SARS-CoV-2 Duotang

SARS-CoV-2 Duotang
Duotang, a genomic epidemiology analyses and mathematical modelling notebook
CAMEO
09 February, 2026
Citation
If you use the VirusSeq Data Portal or Duotang resource, please cite: Gill et al. (2024) Microbial Genomics. doi:10.1099/mgen.0.001293
SARS-CoV-2 In Canada
Introduction
This notebook was built to explore Canadian SARS-CoV-2 genomic and epidemiological data with the aim of investigating viral evolution and spread. It is developed by the CAMEO team (Computational Analysis, Modelling and Evolutionary Outcomes Group) for sharing with collaborators, including public health labs. These analyses are freely available and open source, enabling code reuse by public health authorities and other researchers for their own use.
Canadian genomic and epidemiological data will be regularly pulled from various public sources (see list below) to keep these analyses up-to-date. Only representations of aggregate data will be posted here.
Important limitations
These analyses represent only a snapshot of SARS-CoV-2 evolution in Canada. Only some infections are detected by PCR testing, only some of those are sent for whole-genome sequencing, and not all sequences are posted to public facing repositories. Furthermore, sequencing volumes and priorities have changed during the pandemic, specific variants or populations might be preferentially sequenced at certain times in certain jurisdictions. When possible, these differences in sampling strategies are mentioned but they are not always known. With the arrival of the Omicron wave, many jurisdictions across Canada reached testing and sequencing capacity mid-late December 2021 and thus switched to targeted testing of priority groups (e.g., hospitalized patients, health care workers, and people in high-risk settings). Currently, most jurisdictions are sequencing mainly hospitalized patients or outbreaks, with little population-level random sampling, underestimating case counts and viral diversity.
Thus, interpretation of these plots and comparisons between health regions should be made with caution, considering that the data may not be fully representative. These analyses are subject to frequent change given new data and updated lineage designations.
The last sample collection date is 09 January, 2026
Current SARS-CoV-2 situation
While data on cases and genomic sampling has been limited, the data available to date are consistent with variant XFG driving a wave starting in August that was delayed or extended in some regions into late Fall 2025. Then a combination of XFG subvariants and PQ subvariants continued to extend cases.
Variants of current interest (due to their current/potential growth advantage, mutations of potential functional significance, or spread in other countries):
- PQ.2.1 subvariants (descended from NB.1.8.1), particularly PQ.2.1.3
- PQ.2.8.1, or subvariants (includes RC.1)
- PQ.17
- Subvariants of XFG, a recombinant of two Omicron lineages, LF.7 and LP.8.1.2, particularly XFG.1.1/XFG.1.1.1, of note internationally
There is no clear, single variant driving an increase in the provinces reporting, based on data obtained to date.
We continue to monitor for, but have not recently detected, highly divergent variants in Canada (“saltation” lineages with a sudden increase in number of mutations).
Note reductions or delays in data can cause delays in the detection of new variants and more uncertainty when estimating selection.
We thank the global team of those monitoring variants (such as those posting issues here: https://github.com/cov-lineages/pango-designation/issues), and other SARS-CoV-2 genome analysis tool providers (see List of Useful Tools below), which play a key role in identifying new variants of note.
Sublineages in Canada
There are 349 unique named variants currently circulating in Canada since 2025-09-01 (last 120 days). Please see Pango lineage table for number of sequences per lineage present.
Below is an interactive visualization showing frequencies of ciruclating lineages, sub-divided by major sub-lineages, currently circulating in Canada. A table of lineage frequencies can be downloaded by clicking on the (Frequency Table Download) button.
Tips: Click and drag to zoom, double click to reset. Clicking on an item in the legend will hide it, double clicking an item in legend will hide everything else but that item.
Last 120 days
Last 120 days sublineages starting from 2025-09-01 (Frequency Table Download)
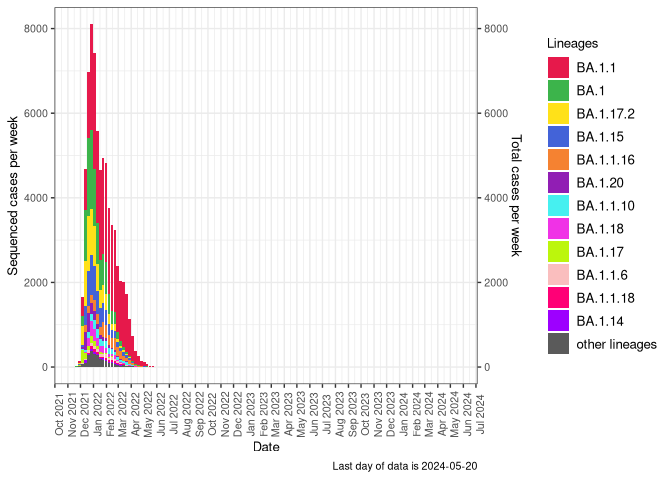
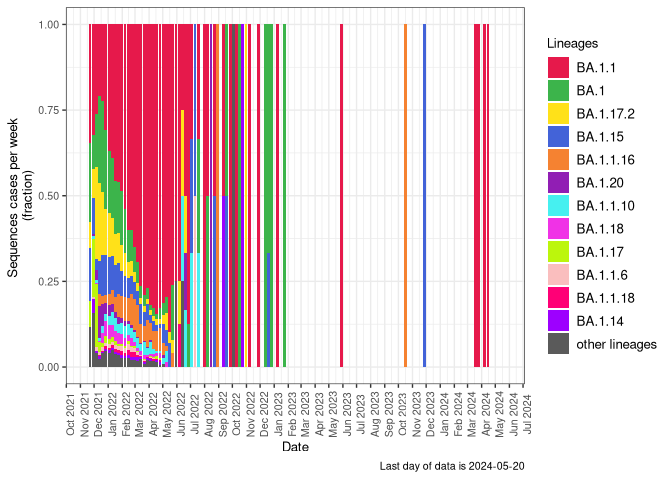
BA.1
BA.1 sublineages (Frequency Table Download)
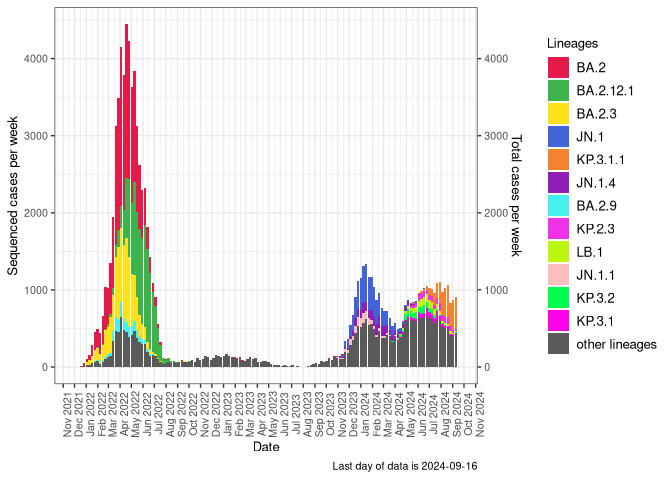
BA.2
BA.2 sublineages (Frequency Table Download)
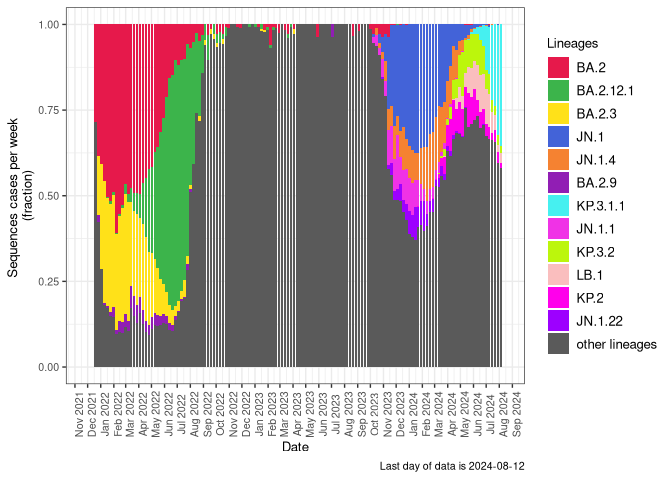
BA.4
BA.4 sublineages (Frequency Table Download)
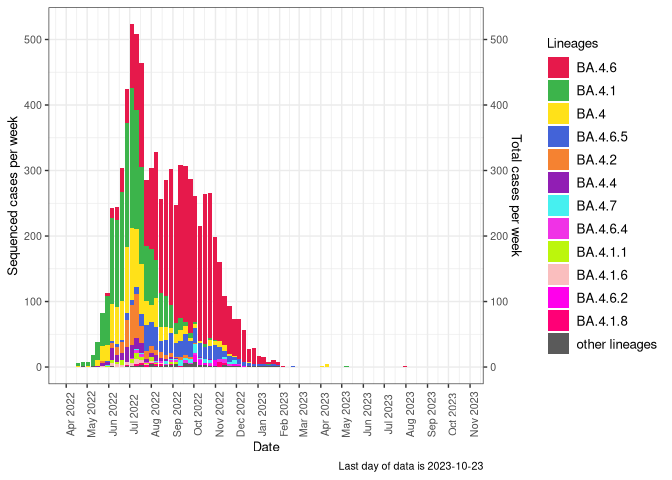
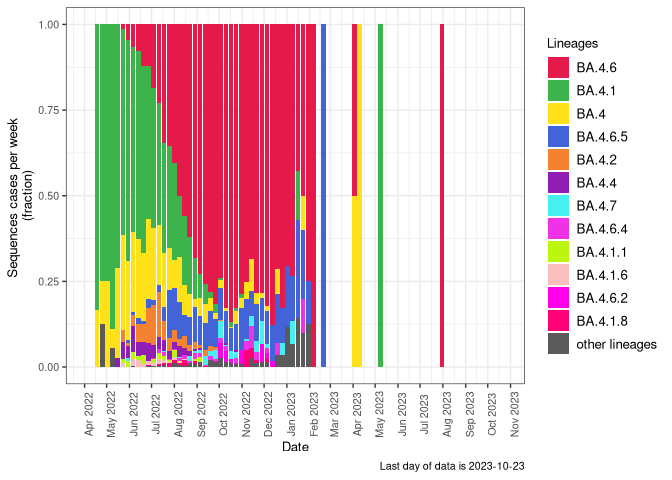
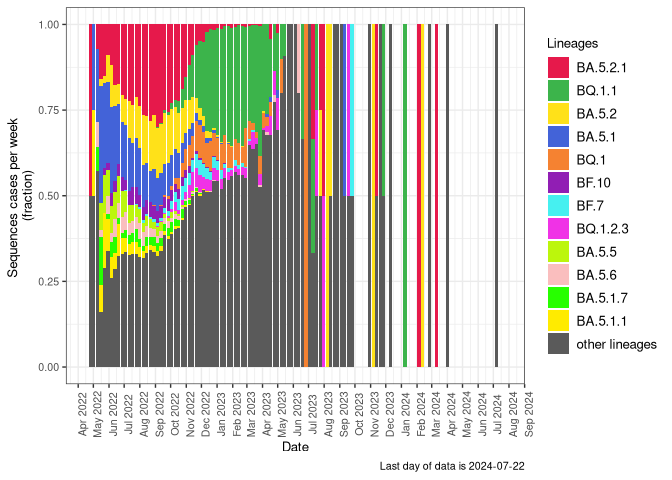
BA.5
BA.5 sublineages (Frequency Table Download)
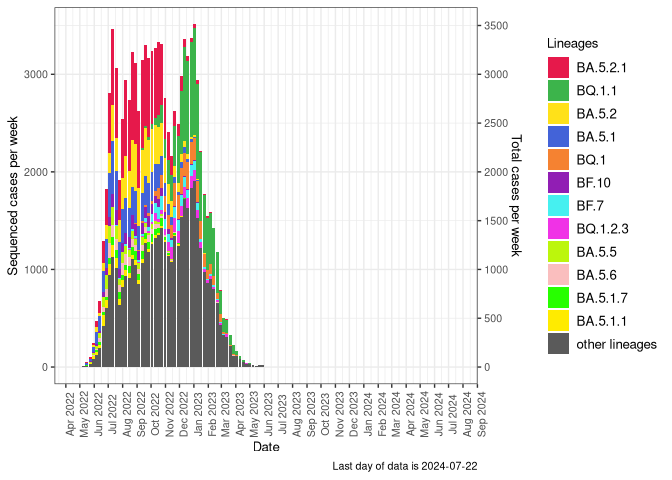
Recombinants
Recombinants sublineages (Frequency Table Download)
## [1] NA
## [1] NASelection on recent variants
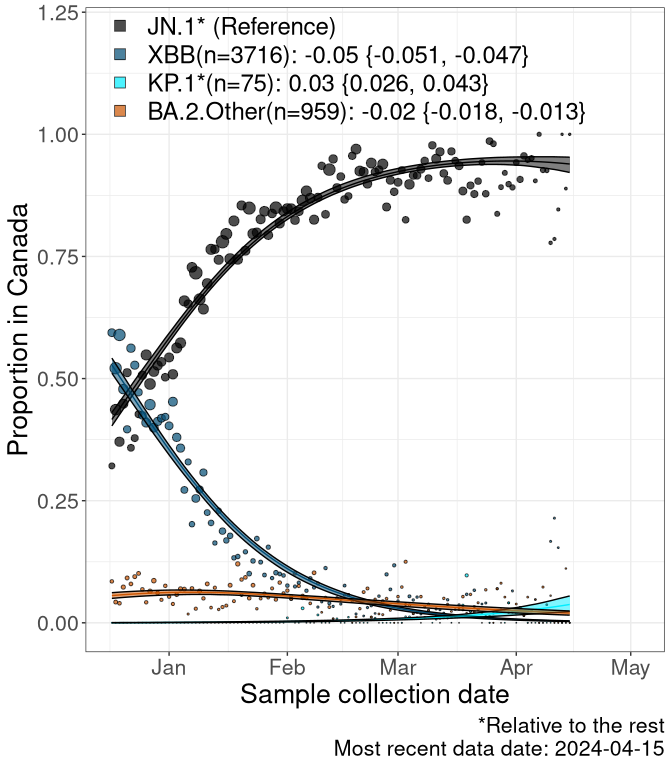
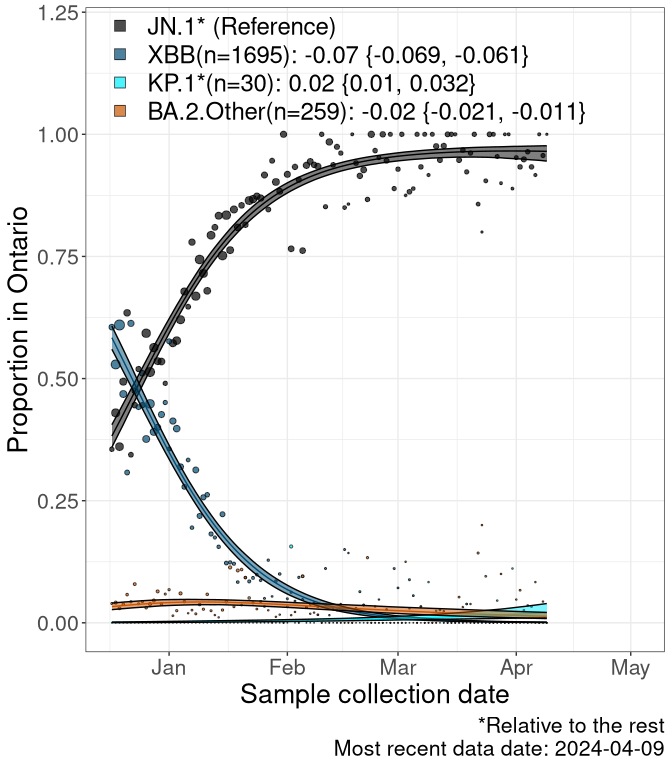
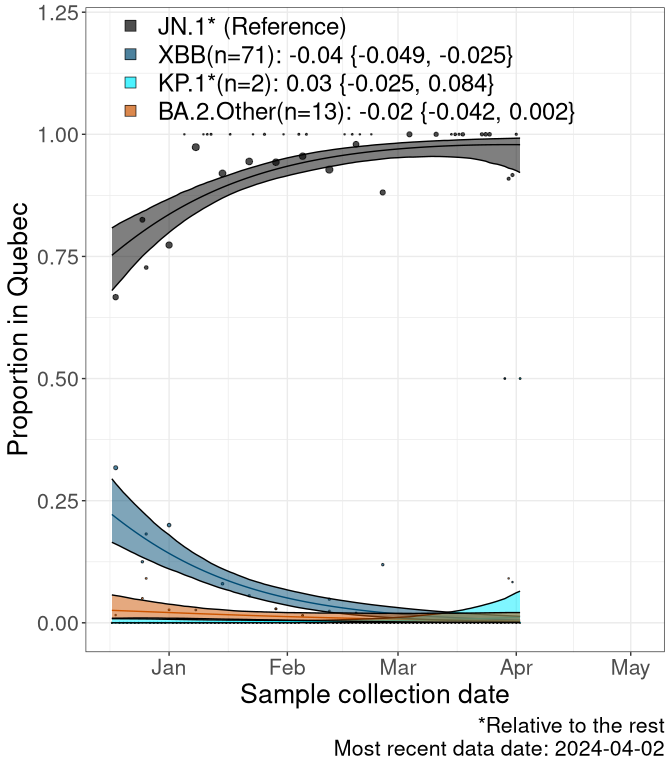
Here we examine the relative rate of spread of the different sublineages of SARS-CoV-2 currently circulating in Canada. Specifically, we determine if a new or emerging lineage has a selective advantage (s), and by how much, against a previously common reference lineage (broad scale (and in the Fastest Growing Lineages section): XFG* and at the fine scale, against XFG.3; see methods for more details about selection and how it is estimated).
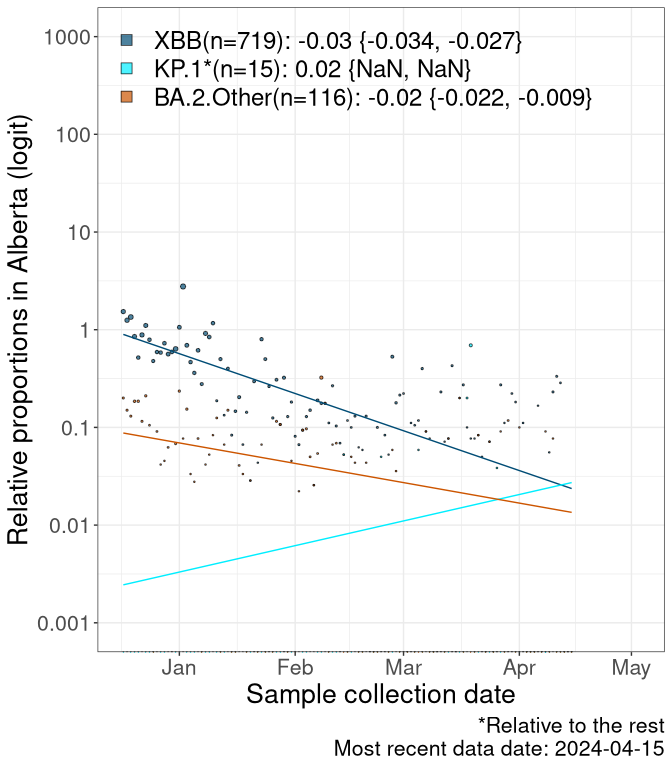
Currently, the major group of SARS-CoV-2 lineages circulating are BA.2.86* variants. At the broad scale, we are now tracking the frequencies of XFG* (as the reference), NB, PQ and other BA.2 lineages (mainly BA.2.86 lineages).
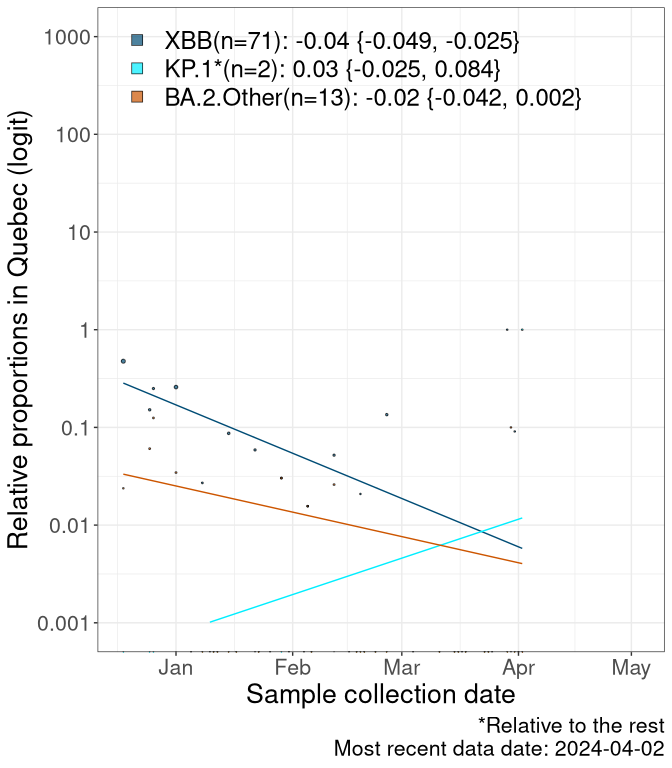
Left plot: y-axis is the proportion of these sub-lineages over time. Right plot: y-axis describes the logit function, log(freq(NB/PQ/Others)/freq(XFG*)), which gives a straight line whose slope is the selection coefficient if selection is constant over time (see methods).
For comparison, Alpha had a selective advantage of s ~ 6%-11% per day over preexisting SARS-CoV-2 lineages, and Delta had a selective advantage of about 10% per day over Alpha.
Caveat: These selection analyses must be interpreted with caution due to the potential for non-representative sampling, lags in reporting, and spatial heterogeneity in prevalence of different sublineages across Canada. Provinces that do not have at least 20 sequences of a lineage during this time frame are not displayed.
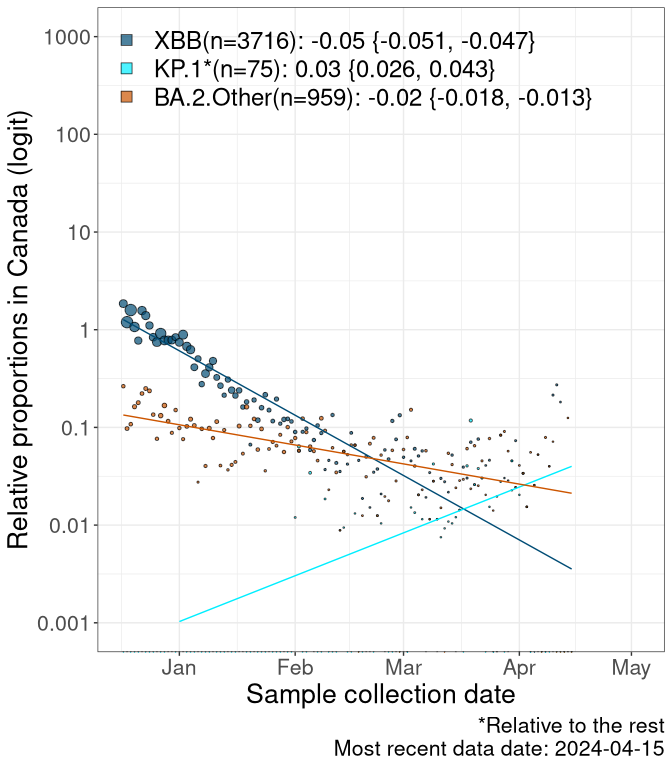
Canada
Canada
BC
British Columbia
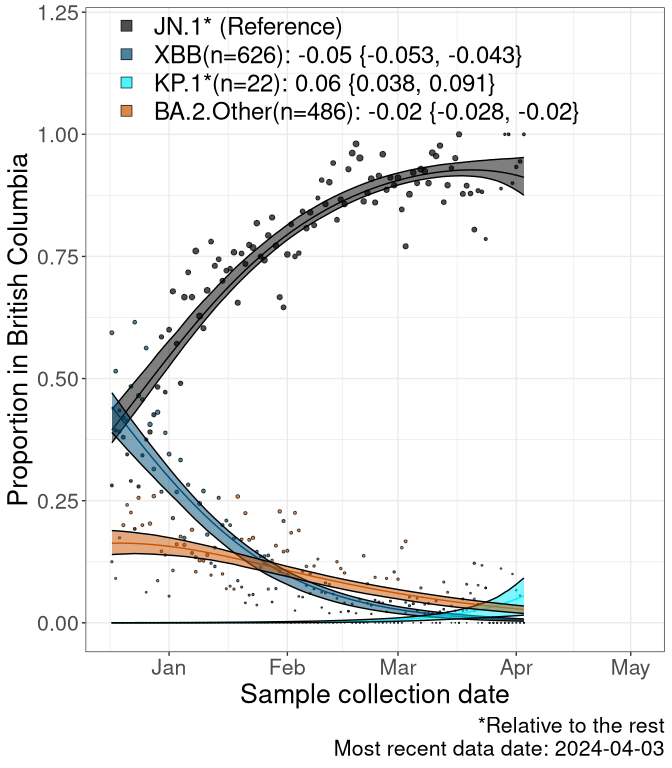
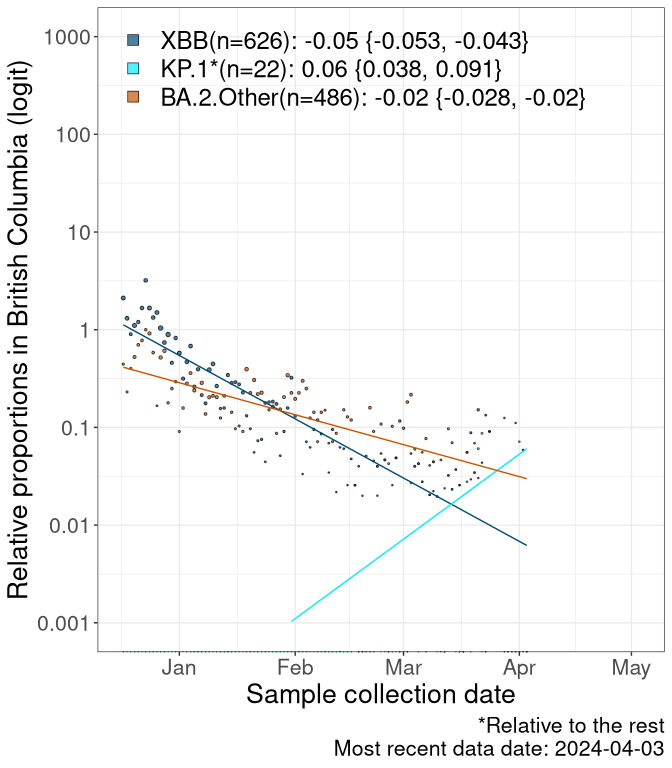
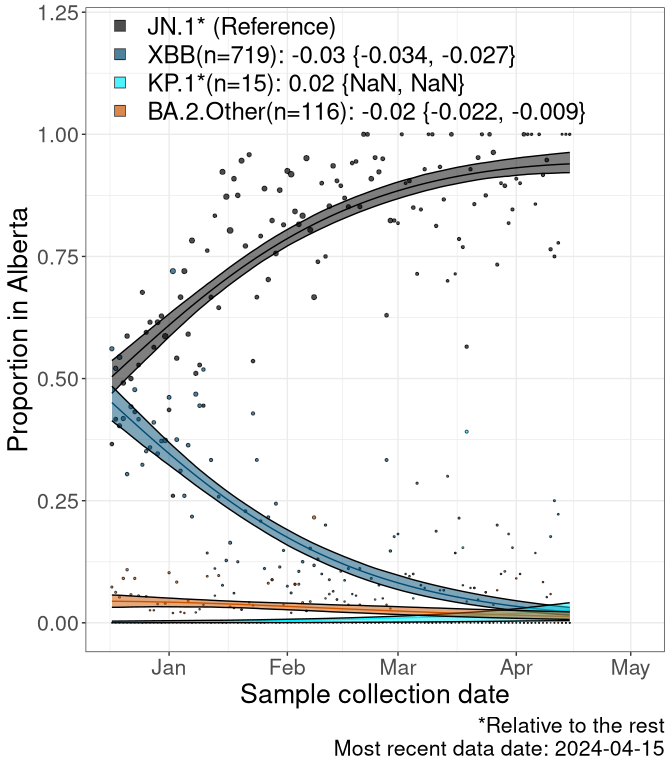
AB
Alberta
SK
Saskatchawan
MB
Manitoba
ON
Ontario
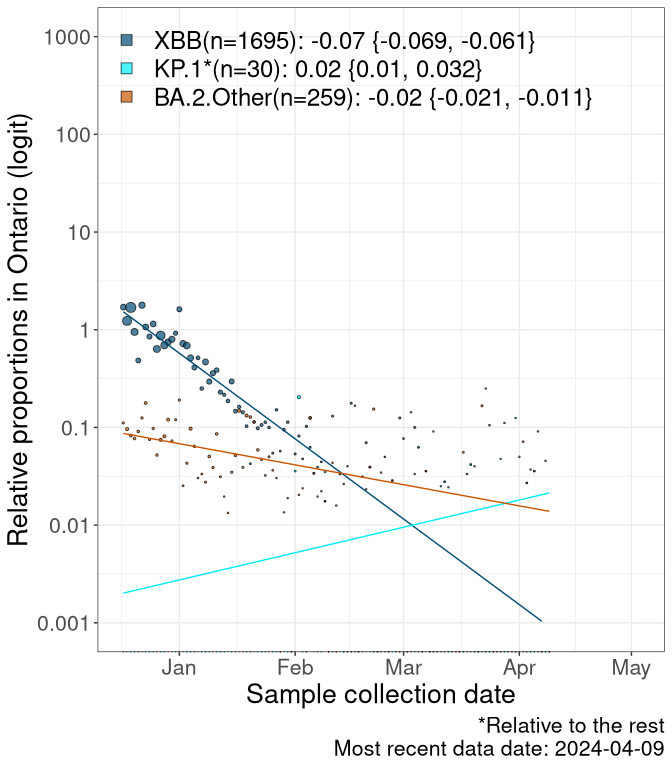
QC
Quebec
NS
Nova Scotia
NB
New Brunswick
NL
Newfoundland and Labrador
NULL
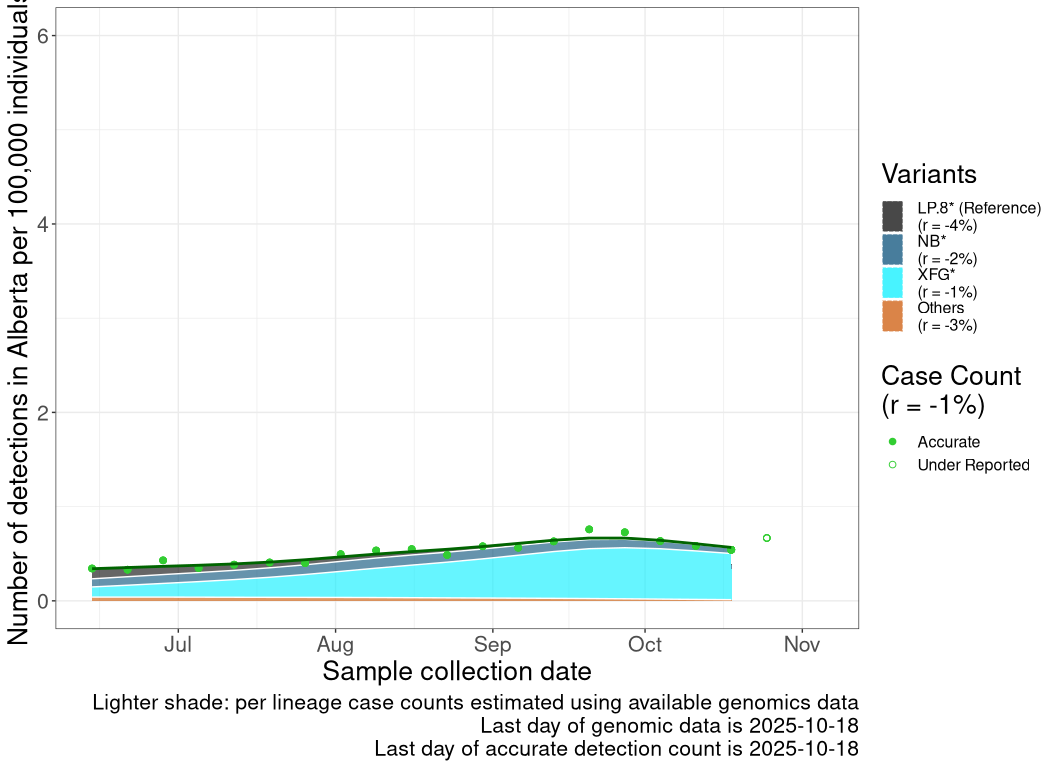
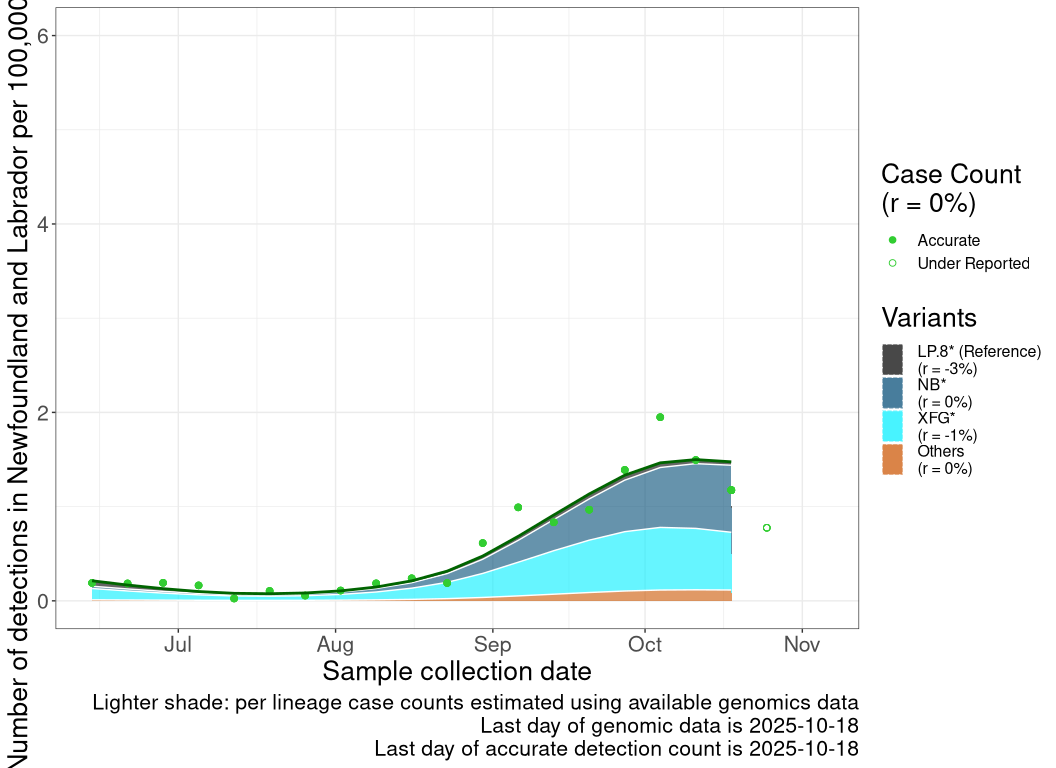
Detection trends by variant
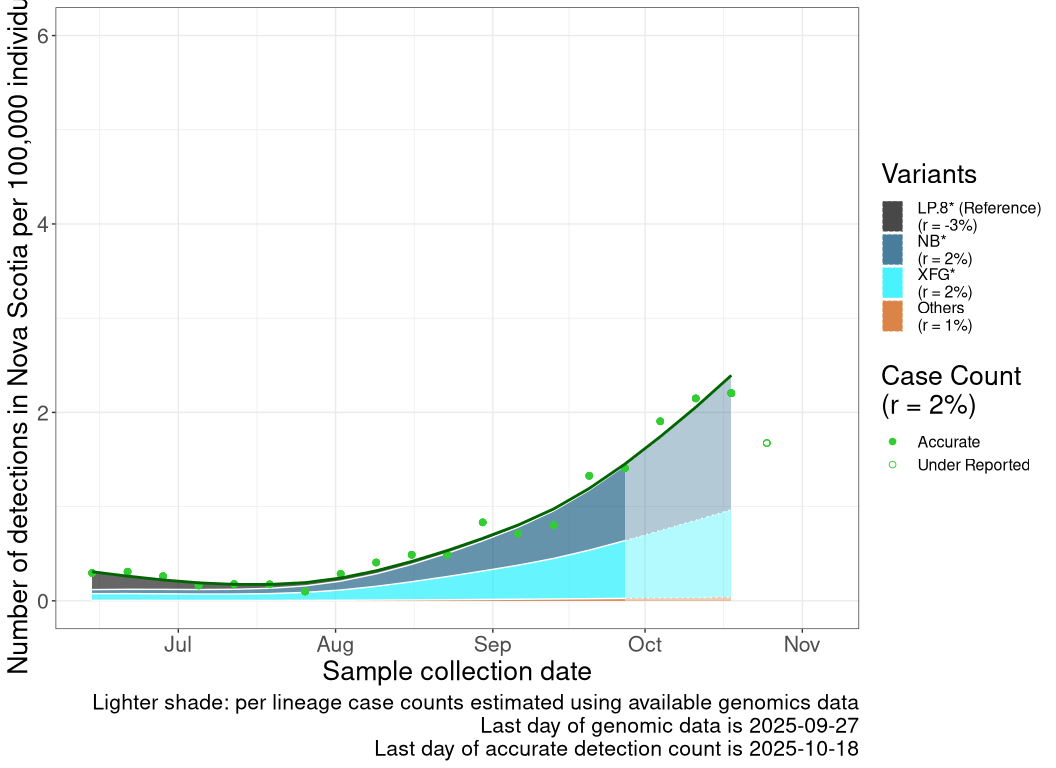
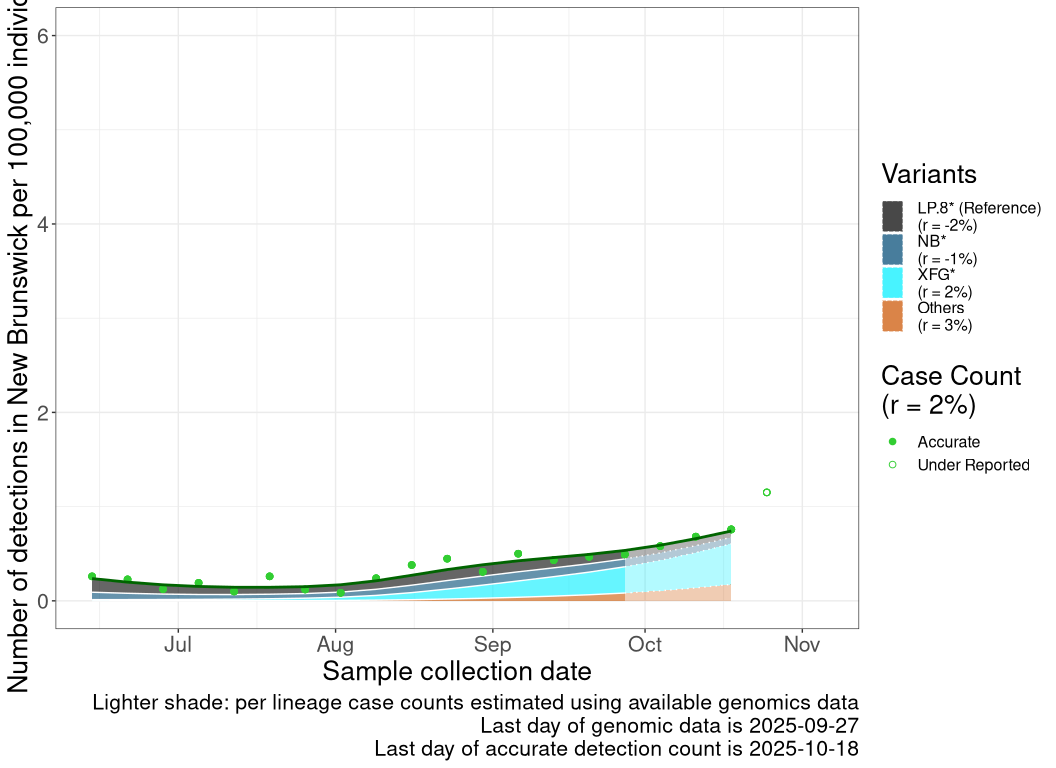
These plots follow the number of detected cases per 100,000 individuals (green dots), ignoring the most recent week (hollow circles), which is generally underestimated as data continue to be gathered. A cubic spline is fit to the log of these case counts to illustrate trends (top curve). The last two days of inferred case counts are then used to estimate the daily exponential growth rate r in COVID-19 cases. The fit from the “Selection on Omicron” section above is used to show how each of the sub-lineages is growing or shrinking, with the corresponding growth rate \(r\) for each sub-lineage on the last two days of inferred case counts. Note that only a small fraction of cases are currently being officially tested, so the y-axis height is underestimated by orders of magnitude (e.g., by 92-fold in BC mid-2022, Skowronski et al. 2022). Thus, graphs should only be used to describe growth trends and not absolute numbers. For detailed methodology including a change in case data source on 13 July 2024, please see the methods section in the appendix..
Canada
Canada
BC
British Columbia
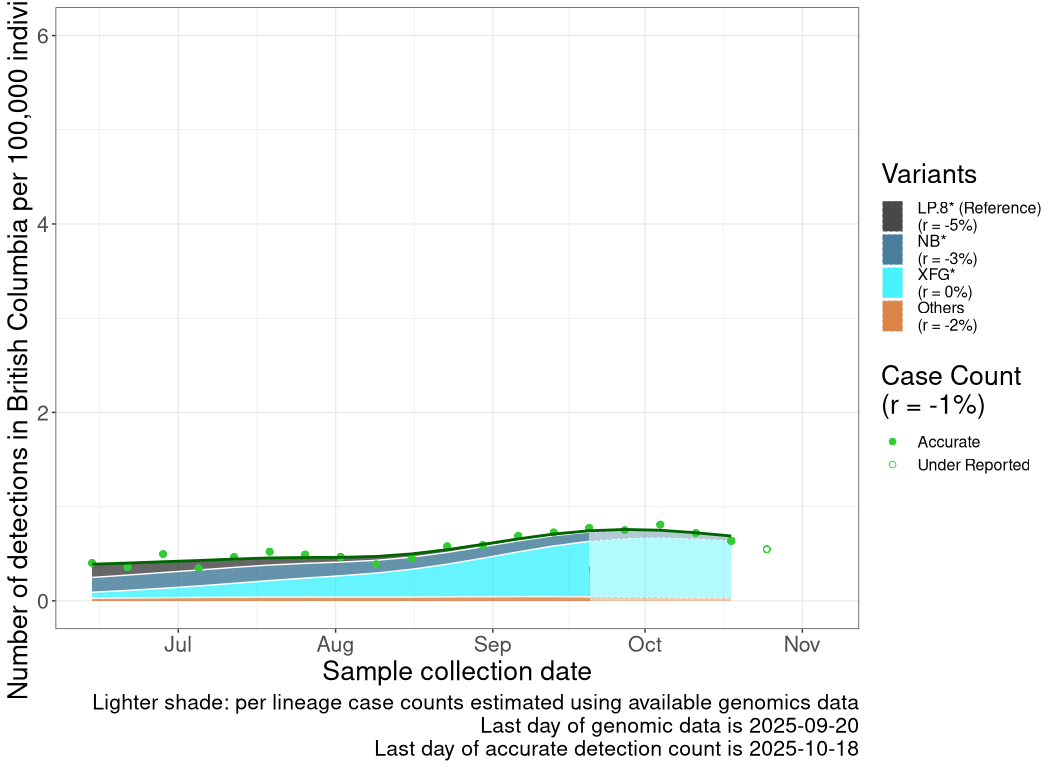
AB
Alberta
SK
Saskatchawan
MB
Manitoba
ON
Ontario
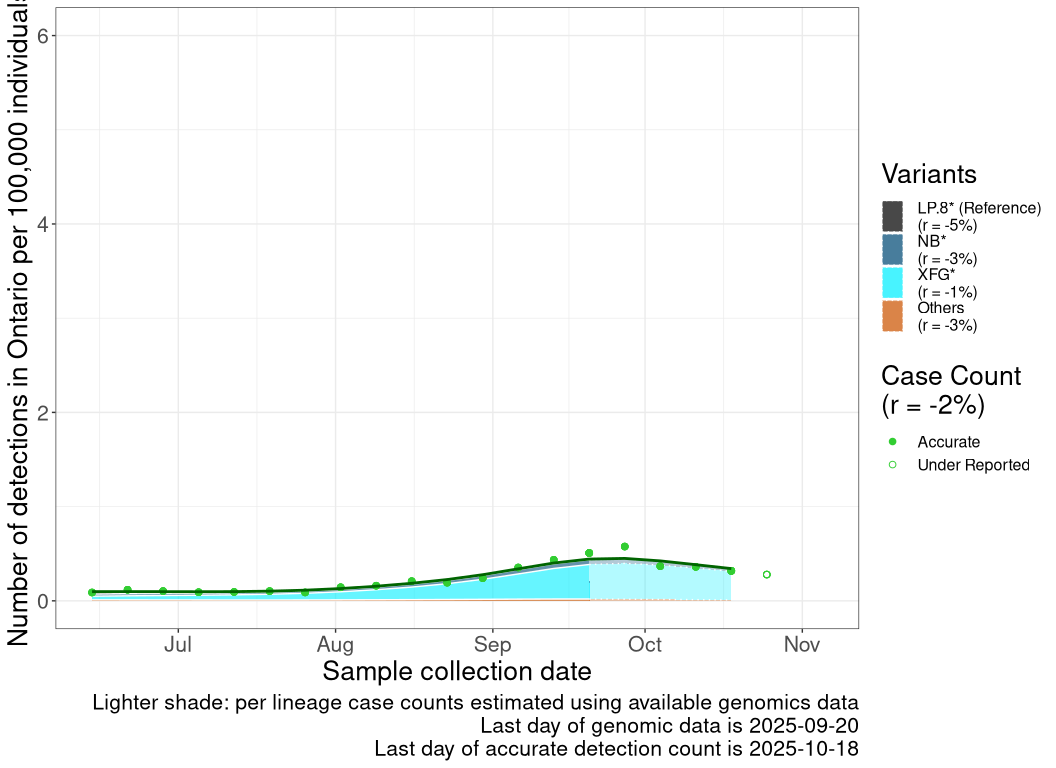
QC
Quebec
NS
Nova Scotia
NB
New Brunswick
NL
Newfoundland and Labrador
NULL
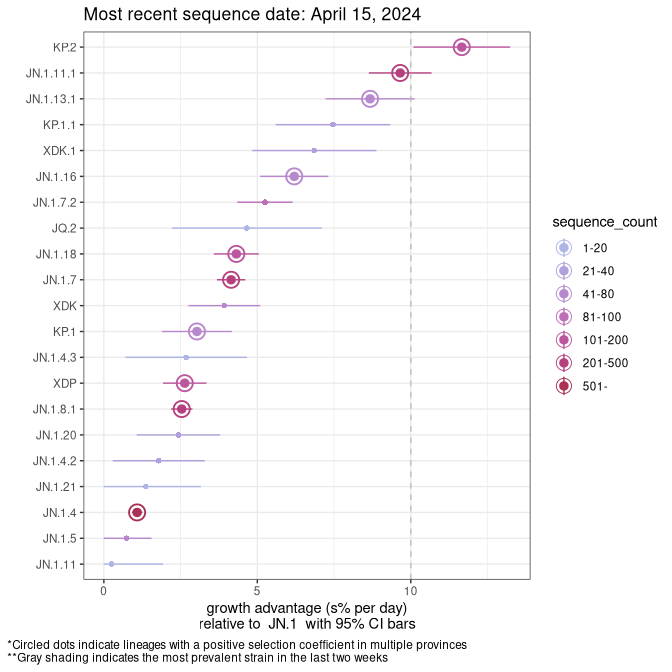
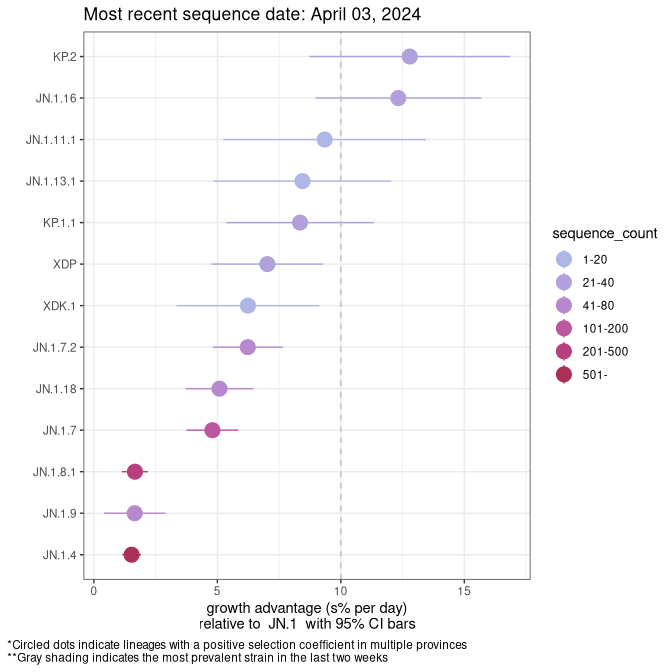
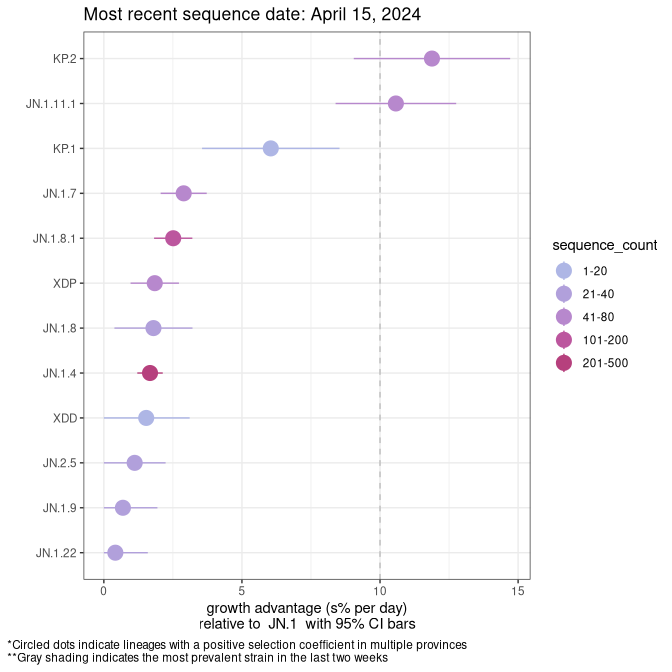
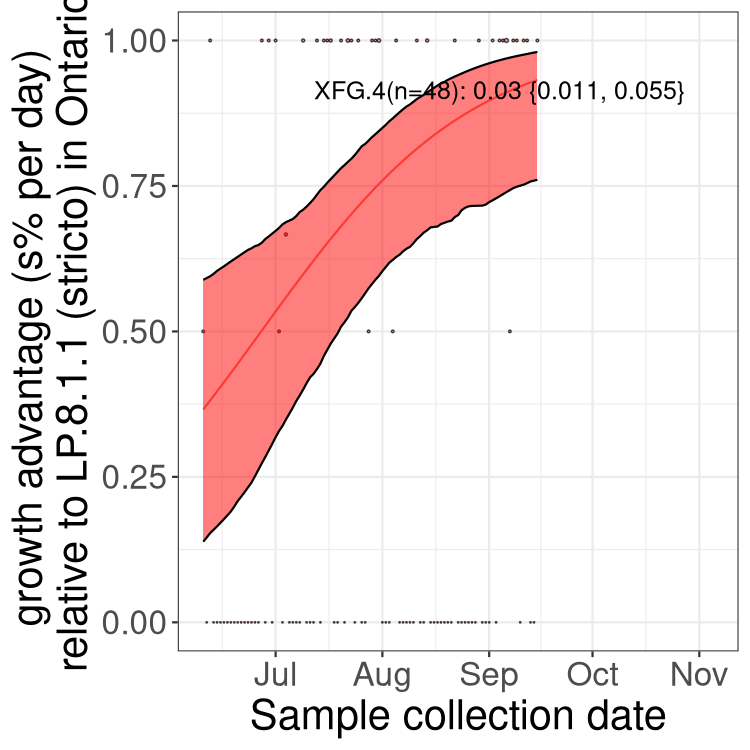
Fastest growing lineages
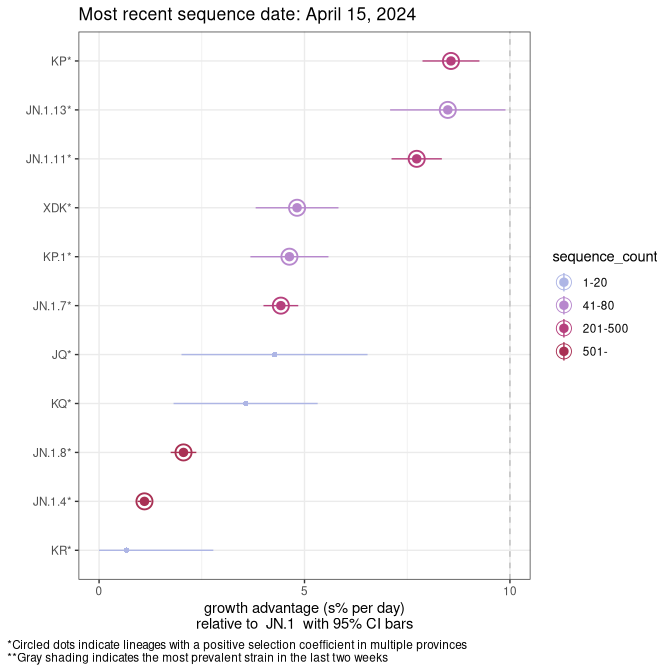
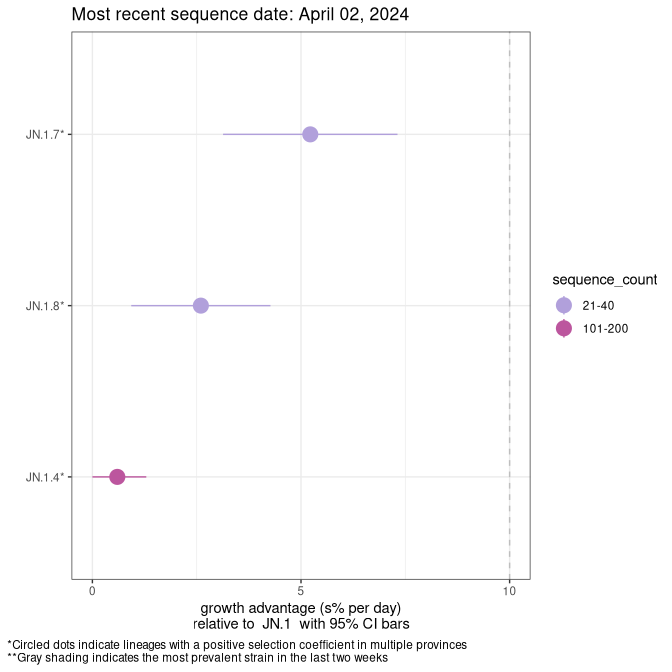
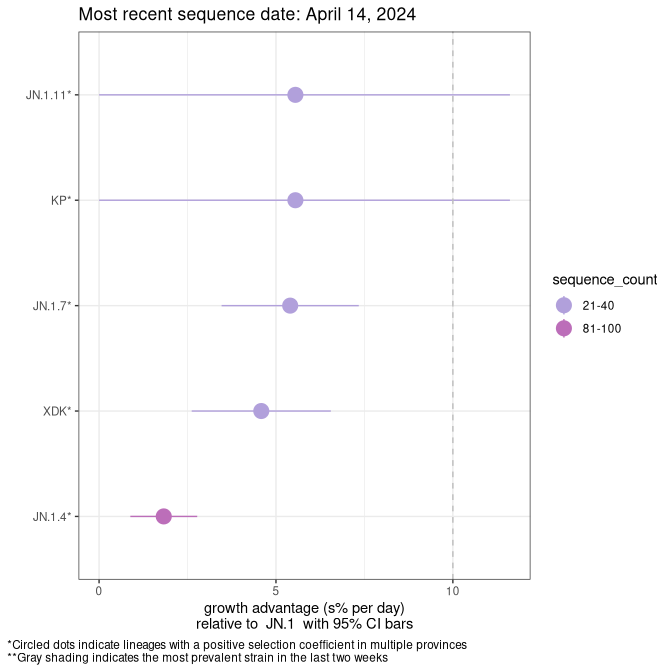
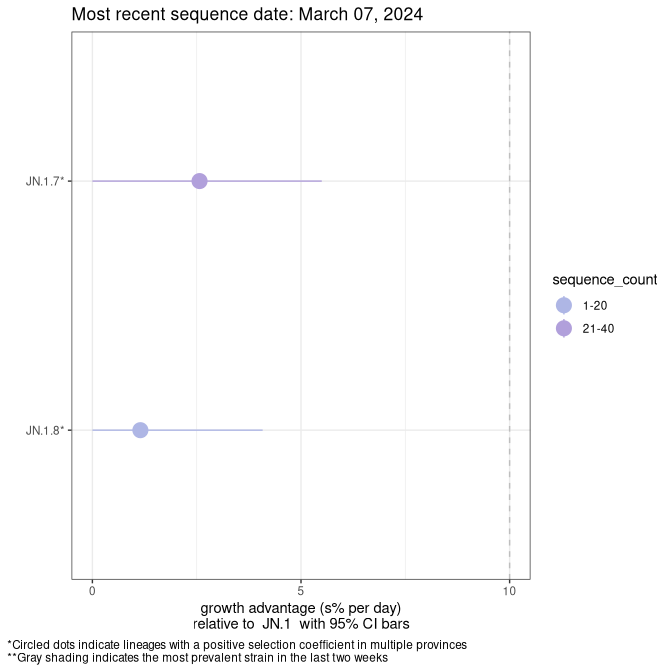
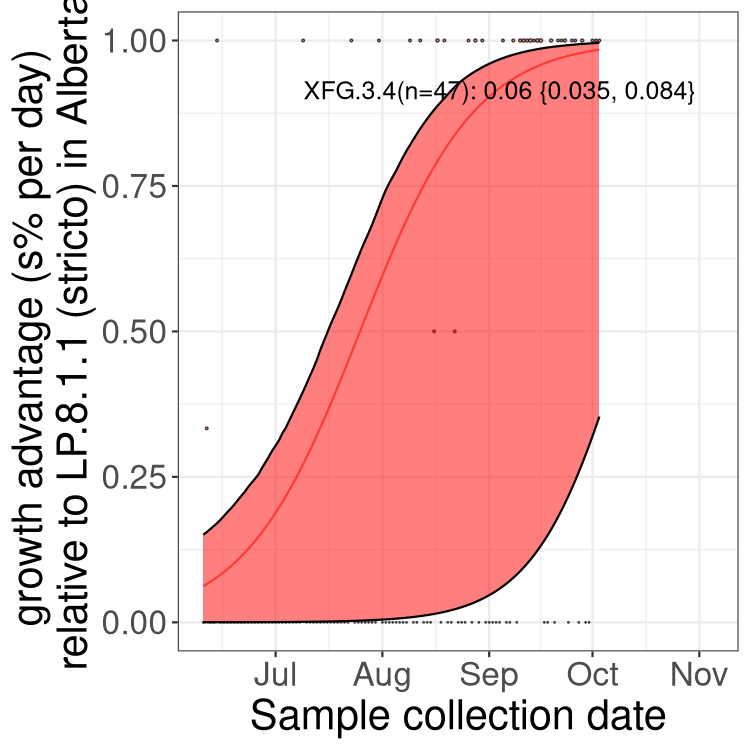
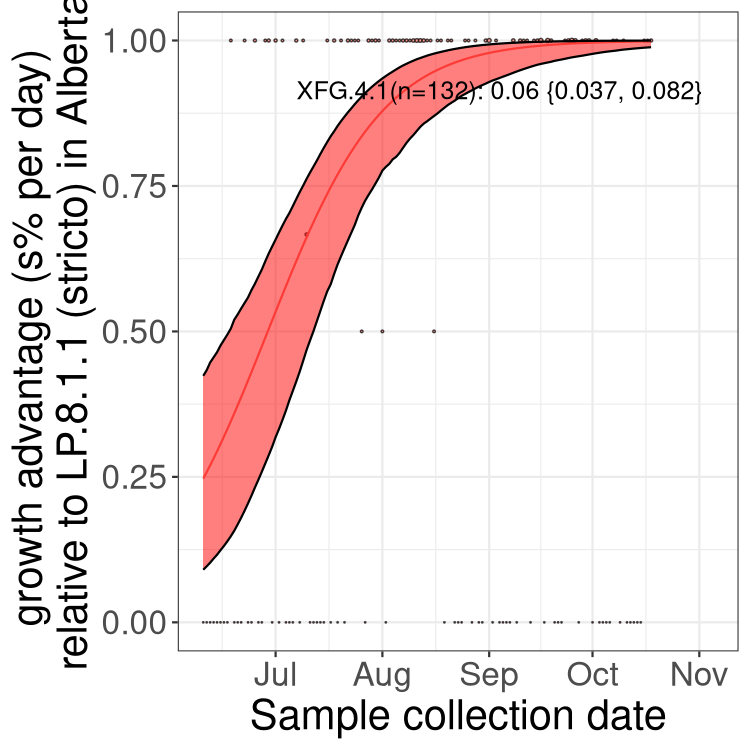
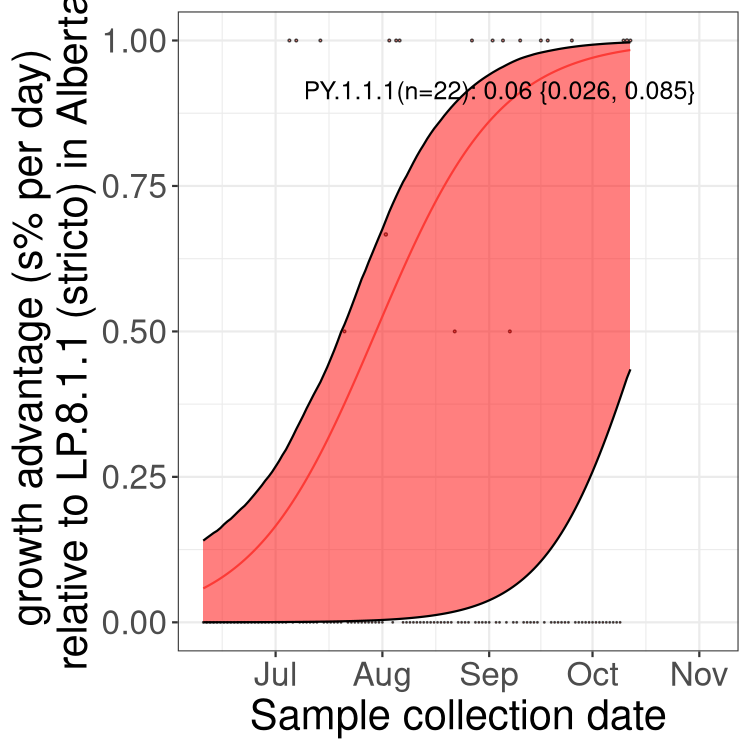
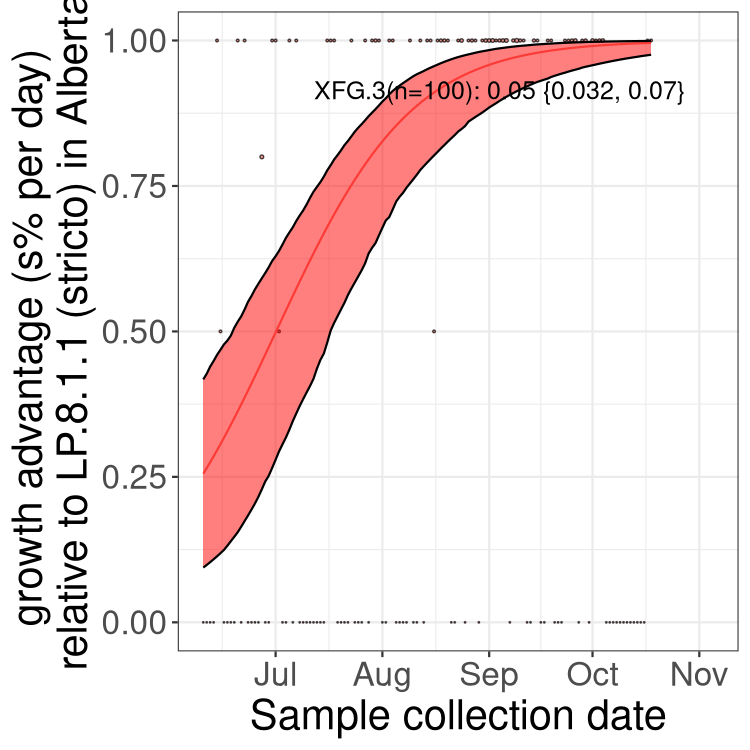
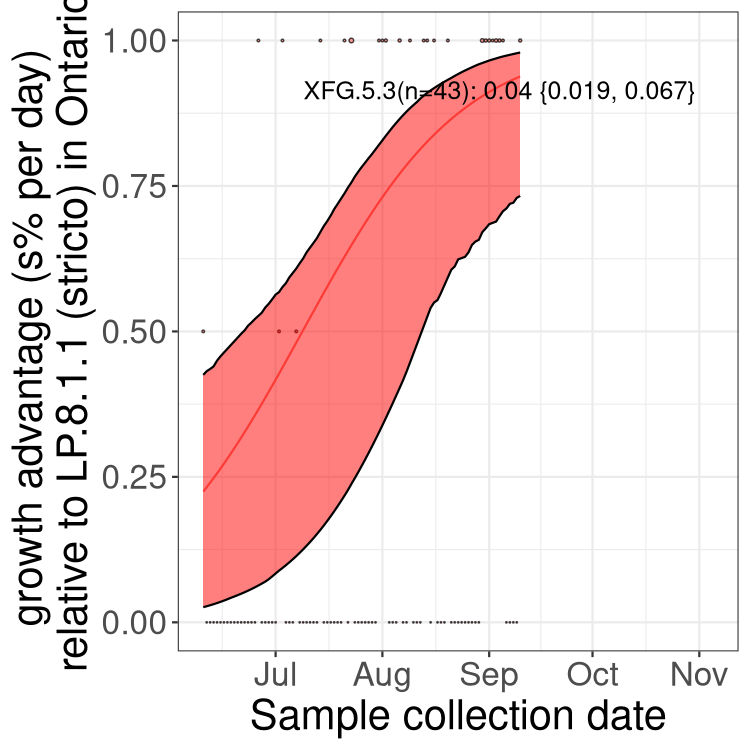
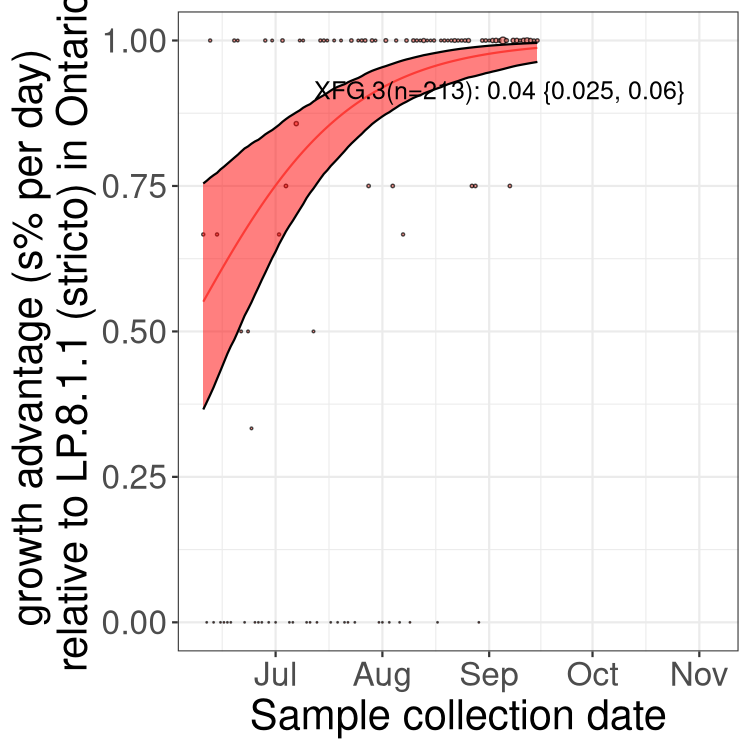
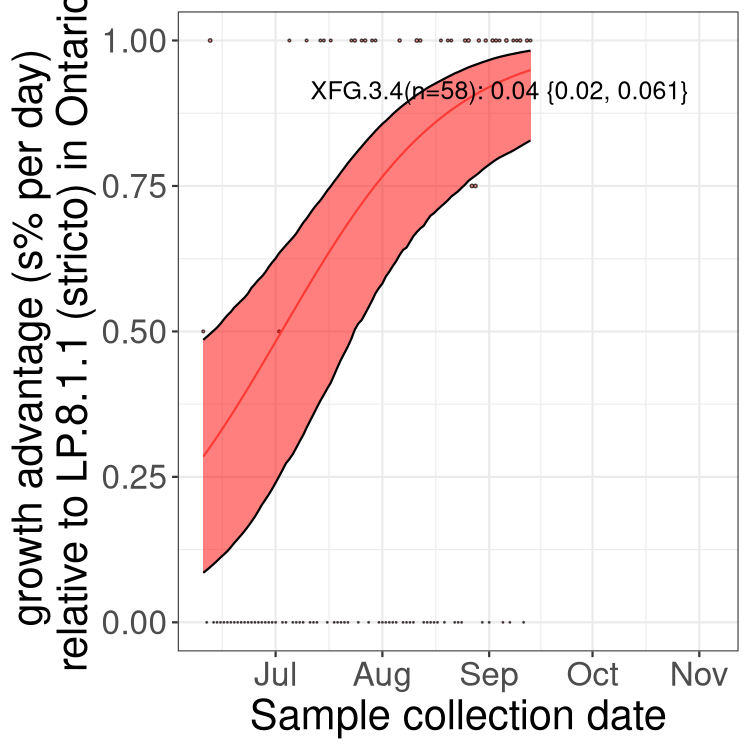
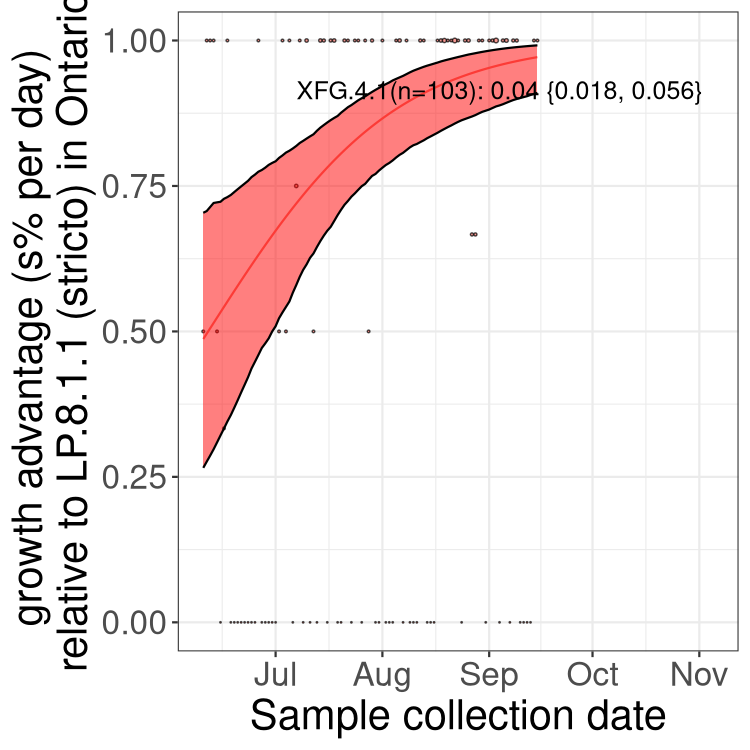
Here we show the selection estimates and their 95% confidence intervals for SARS-CoV-2 lineages with more than 10 sequences in present in a region since 2025-09-01, and with enough data to estimate the confidence interval. Each selection estimate measures the growth rate relative to XFG.3 stricto (i.e., sequences designated as XFG.3 and not its descendants). Plots showing the change in variant frequency over time in Canada as a whole are given below for lineages with more than 50 sequences. For Canada-wide plot, a dot with a circle border indicates lineages with a positive selection coefficient in multiple provinces. The most prevelant lineage in the last two weeks is highlighted in grey. A table of the selection estimates is available for download below.
Growth advantage of 0-5% corresponds to doubling times of more than two weeks, with 5-10% reflecting one to two week doubling times and over 10% representing significant growth of less than one week doubling time. Note that estimating selection of sub-variants with low sequence counts (points with less than 100 counts) is prone to error, such as mistaking one-time super spreader events or pulses of sequence data from one region as selection. Estimates with lower sequence counts in one region should be considered as very preliminary.
Plot (stricto)
This plot highlights single lineages that are growing fastest.
Canada
Plot single lineages in Canada *
BC
Plot single lineages in British Columbia
AB
Plot single lineages in Alberta
SK
Plot single lineages in Saskatchawan
MB
Plot single lineages in Manitoba
ON
Plot single lineages in Ontario
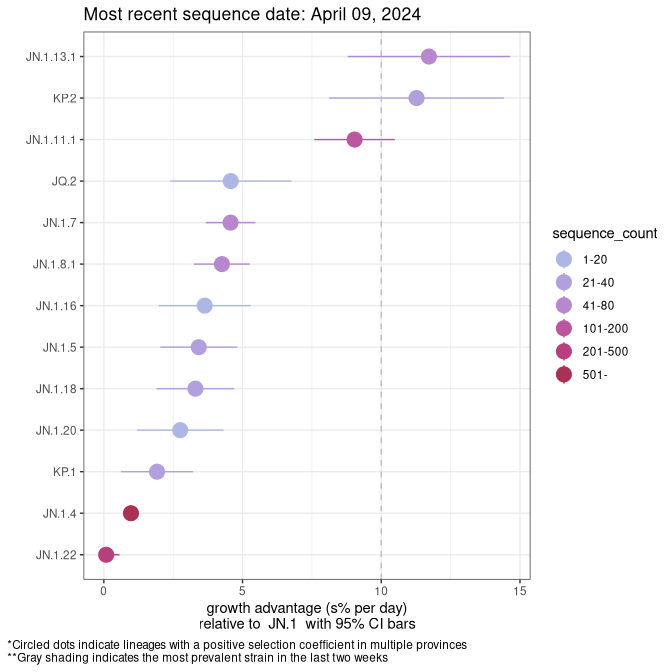
QC
Plot single lineages in Quebec
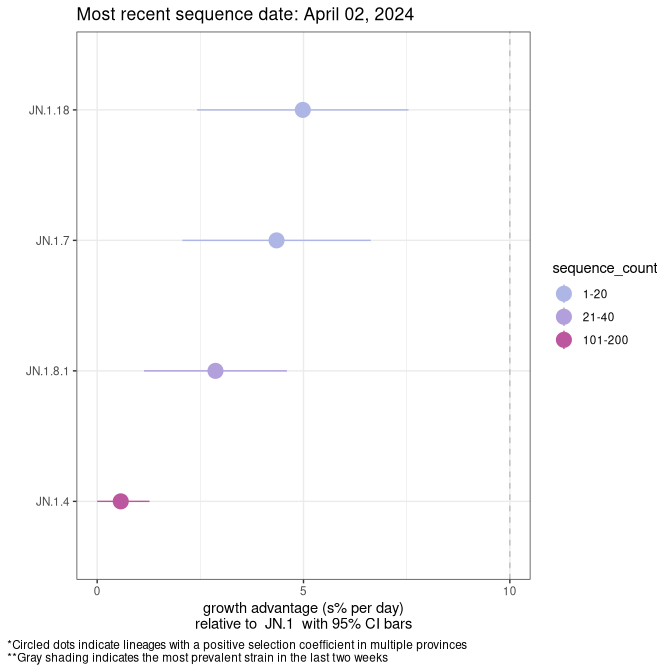
NS
Plot single lineages in Nova Scotia
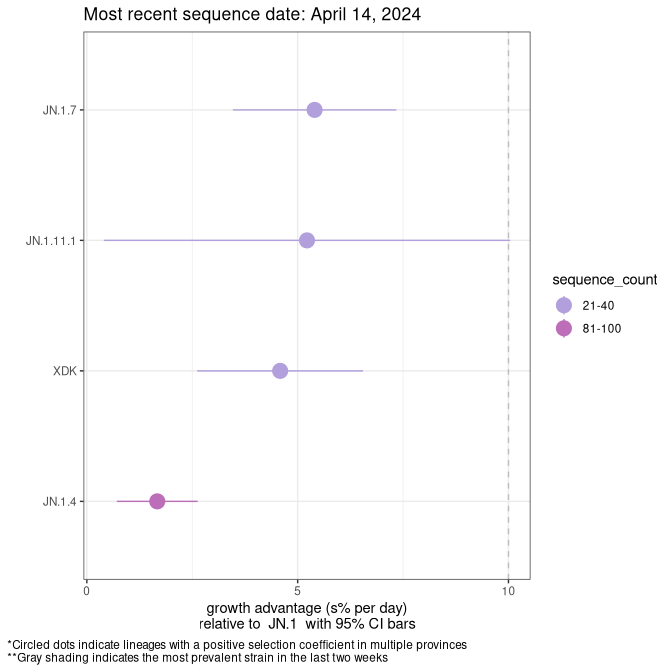
NB
Plot single lineages in New Brunswick
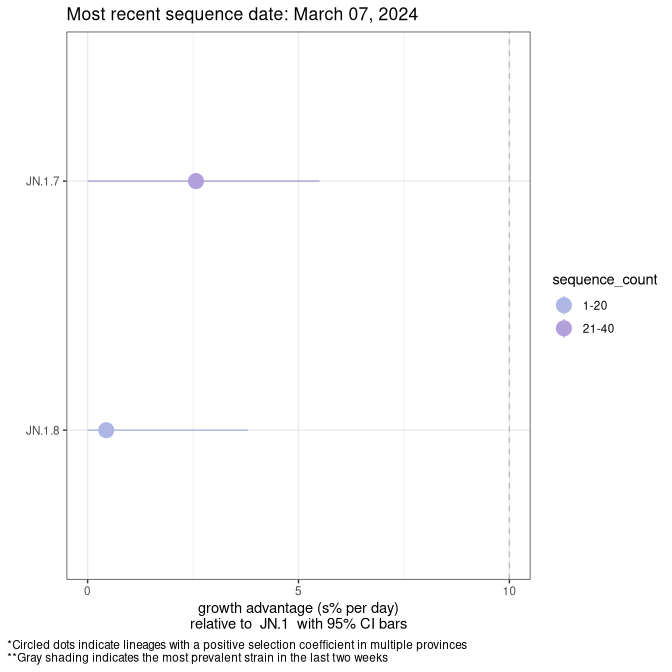
NL
Plot single lineages in Newfoundland and Labrador
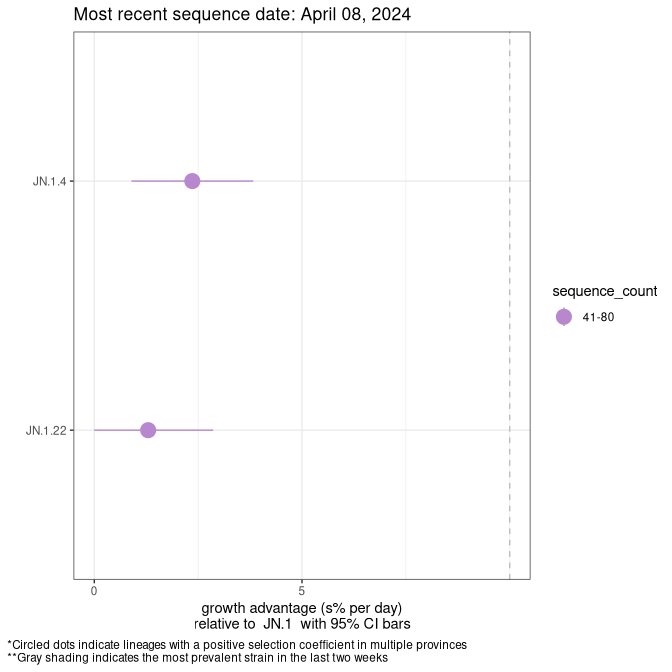
Plot (non stricto)
This plot highlights the groups of related lineages that are growing fastest (e.g., JN.1* is the monophyletic clade that includes JN.1.7 and all other JN.1 sublineages, excluding recombinants.
Canada
Plot single lineages in Canada
BC
Plot single lineages in British Columbia
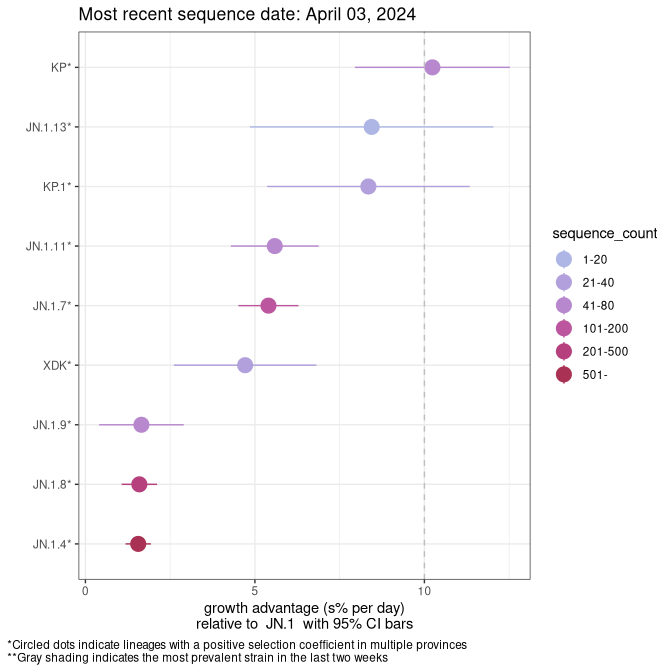
AB
Plot single lineages in Alberta
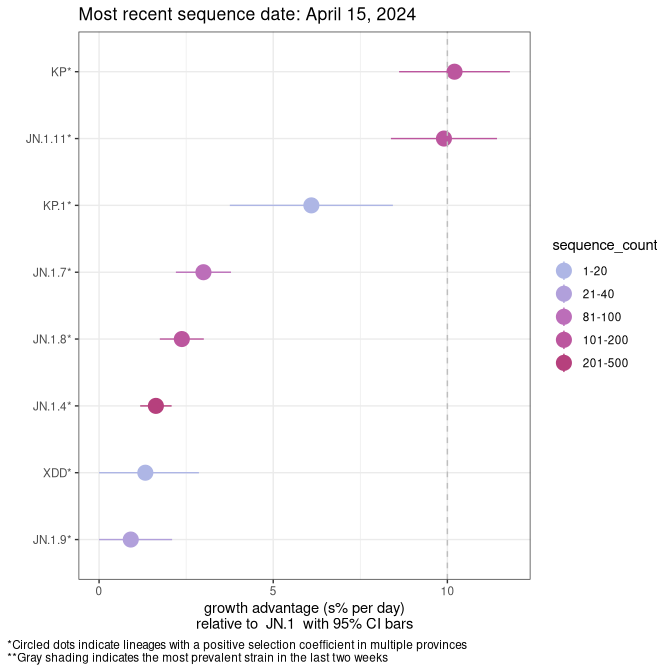
SK
Plot single lineages in Saskatchawan
MB
Plot single lineages in Manitoba
ON
Plot single lineages in Ontario
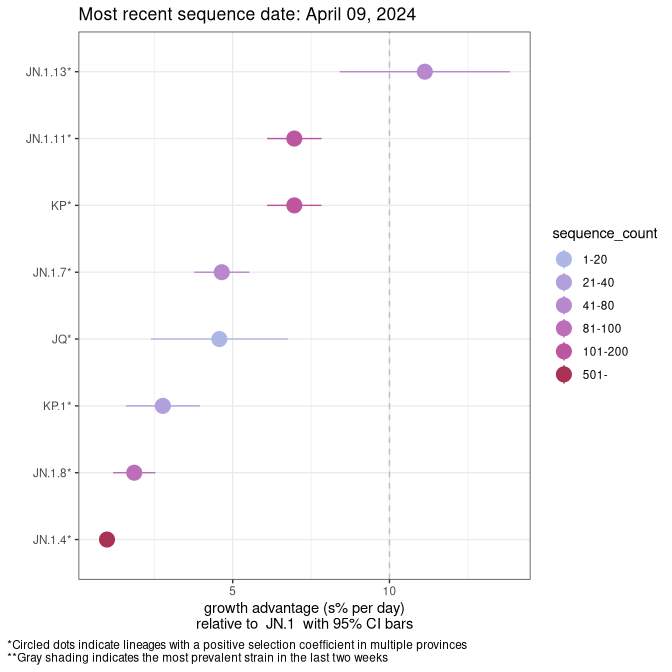
QC
Plot single lineages in Quebec
NS
Plot single lineages in Nova Scotia
NB
Plot single lineages in New Brunswick
NL
Plot single lineages in Newfoundland and Labrador
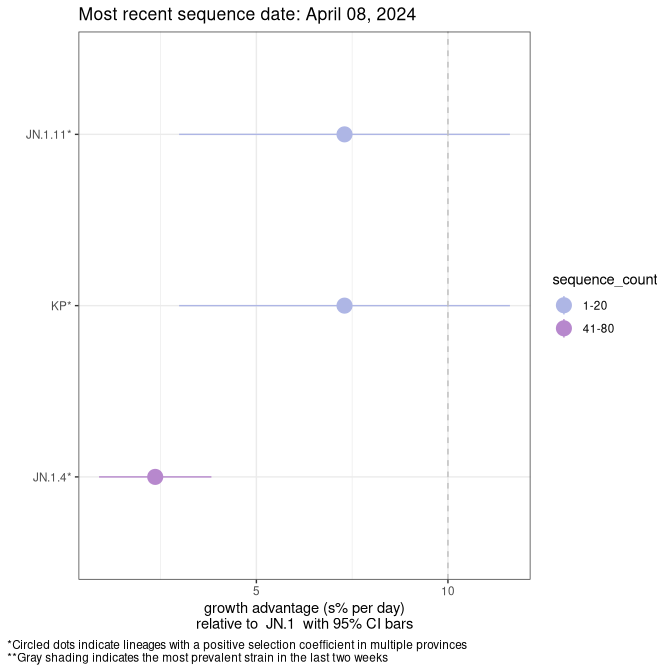
Table of all the selection estimates
Sublineages selection
BA.2 sublineages
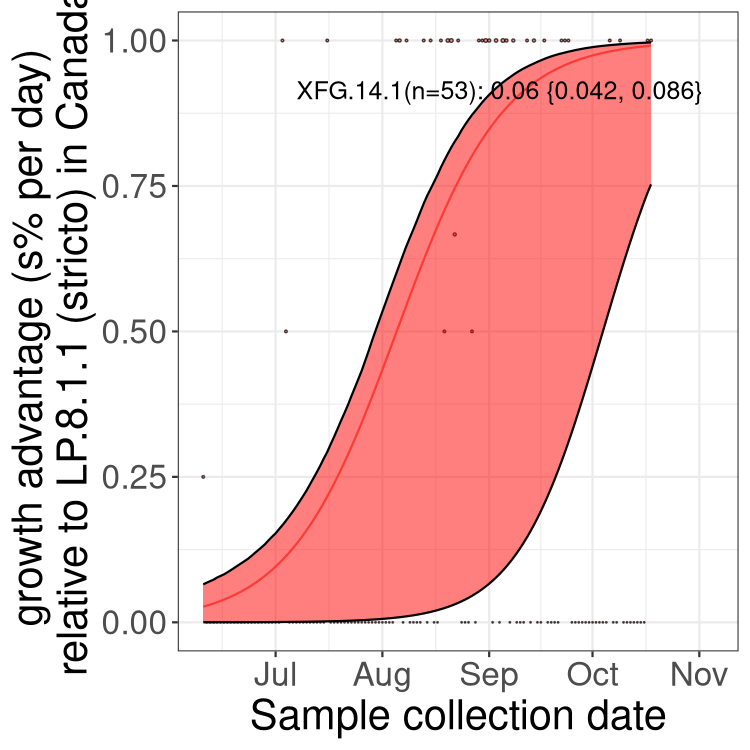
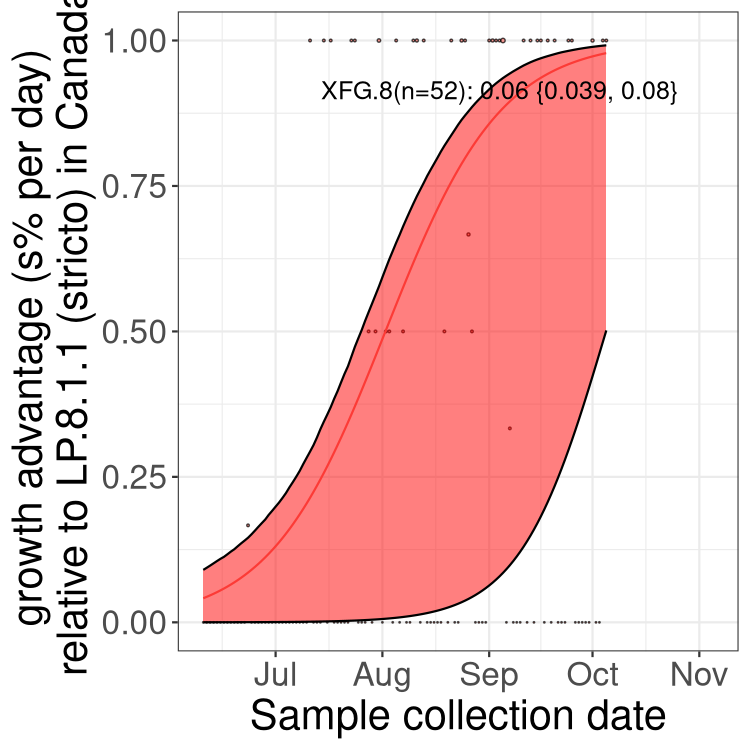
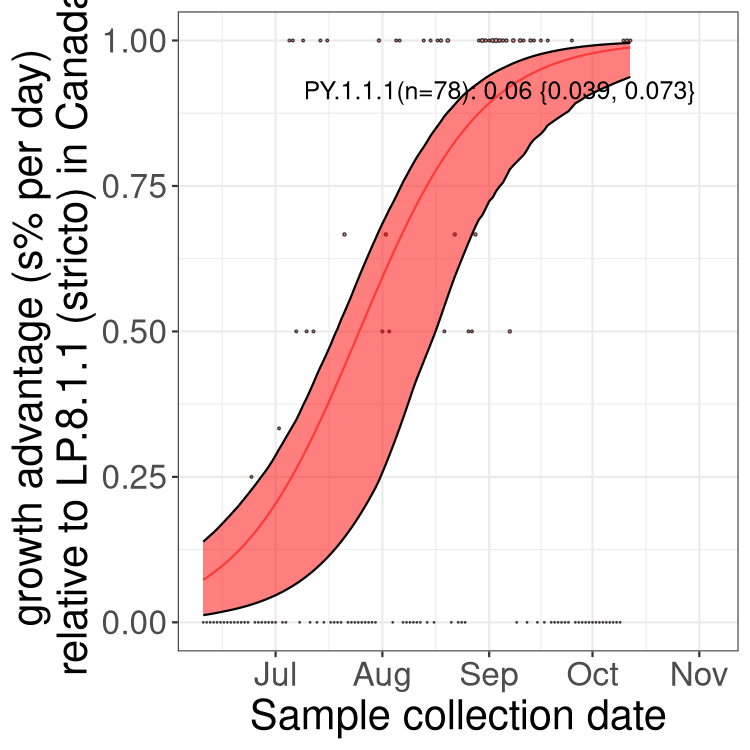
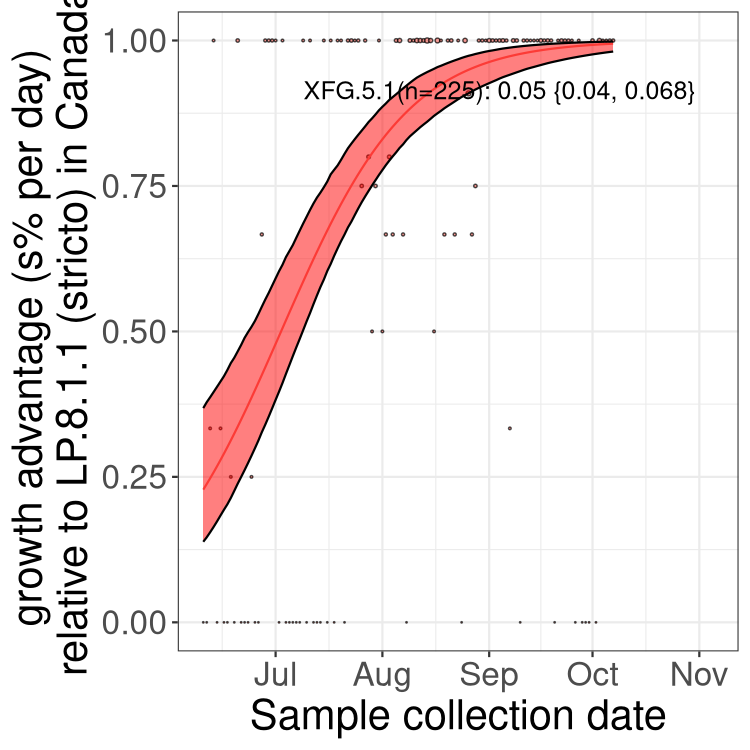
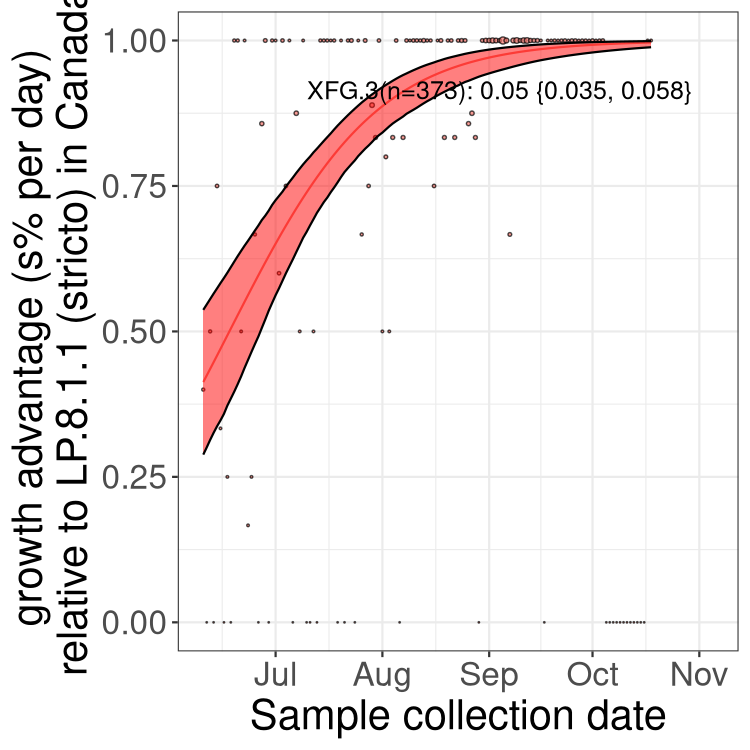
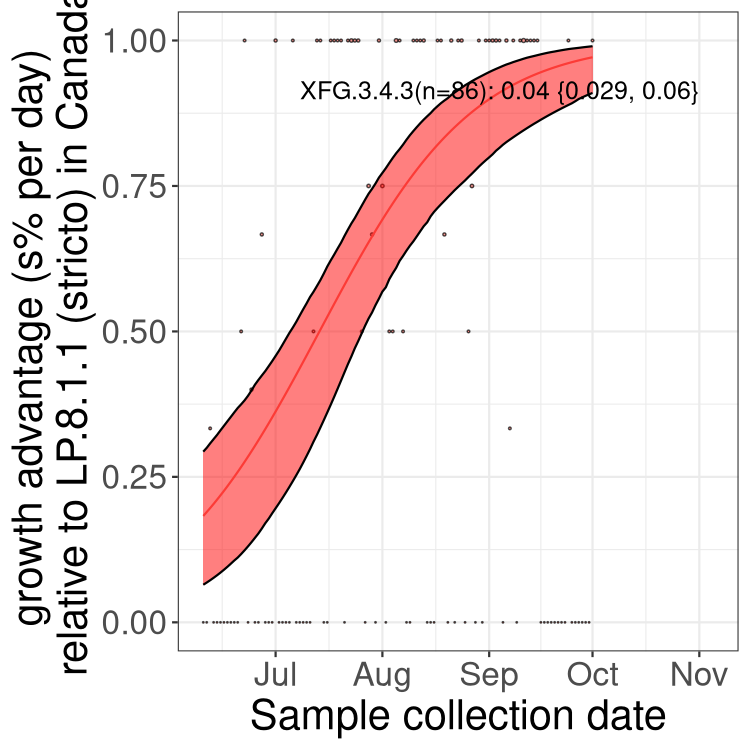
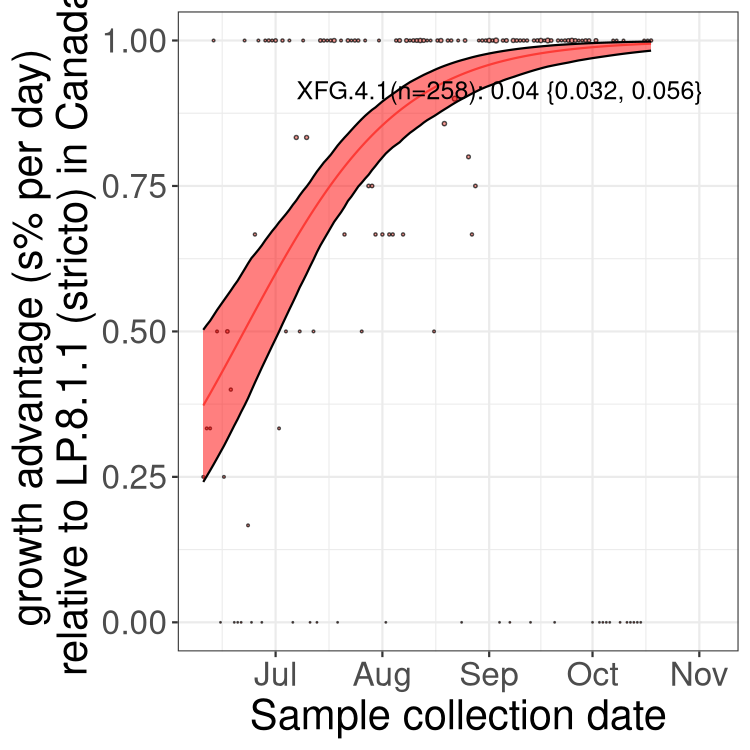
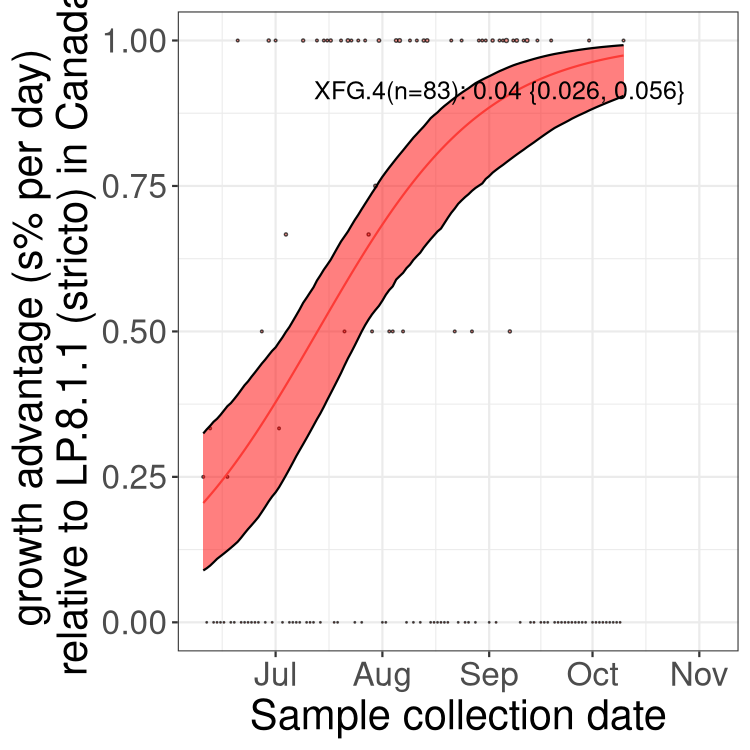
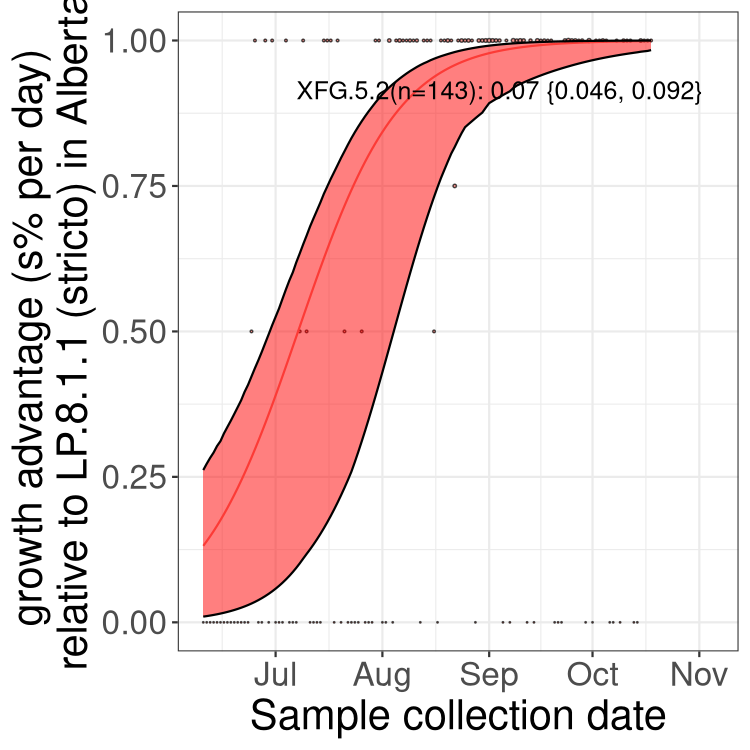
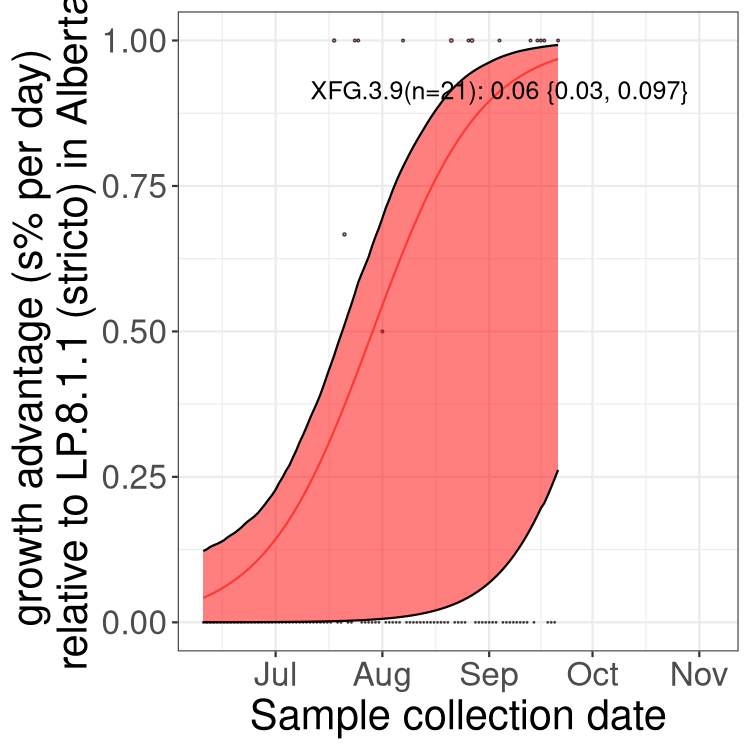
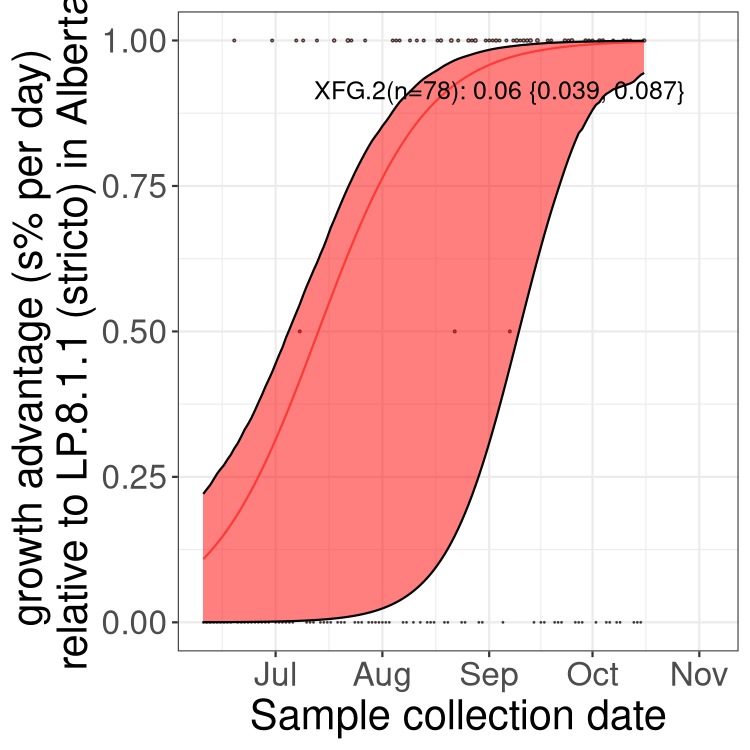
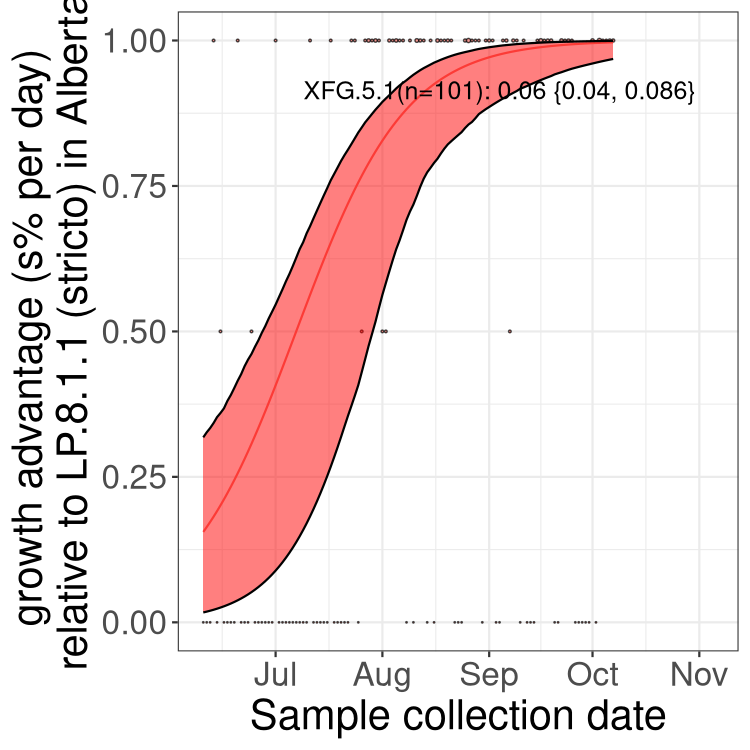
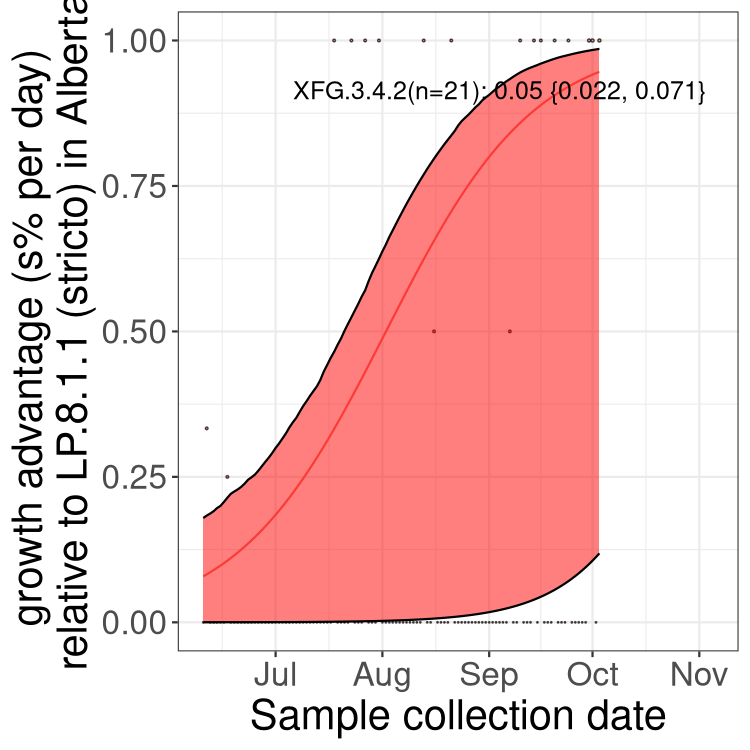
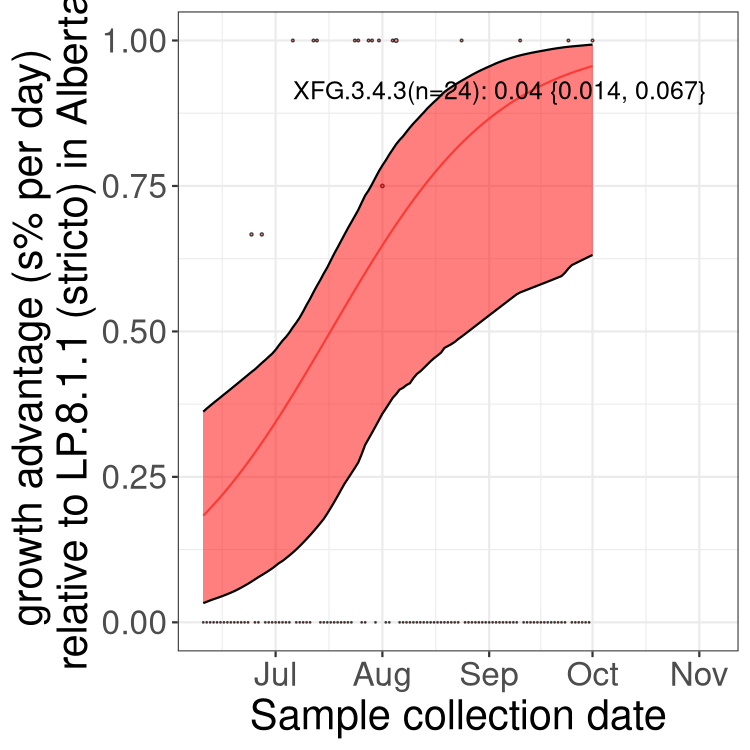
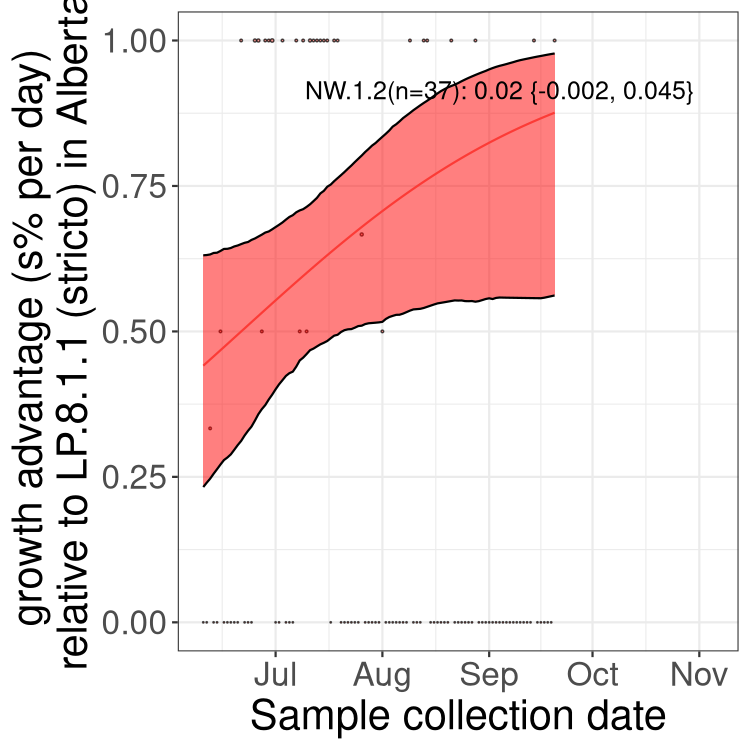
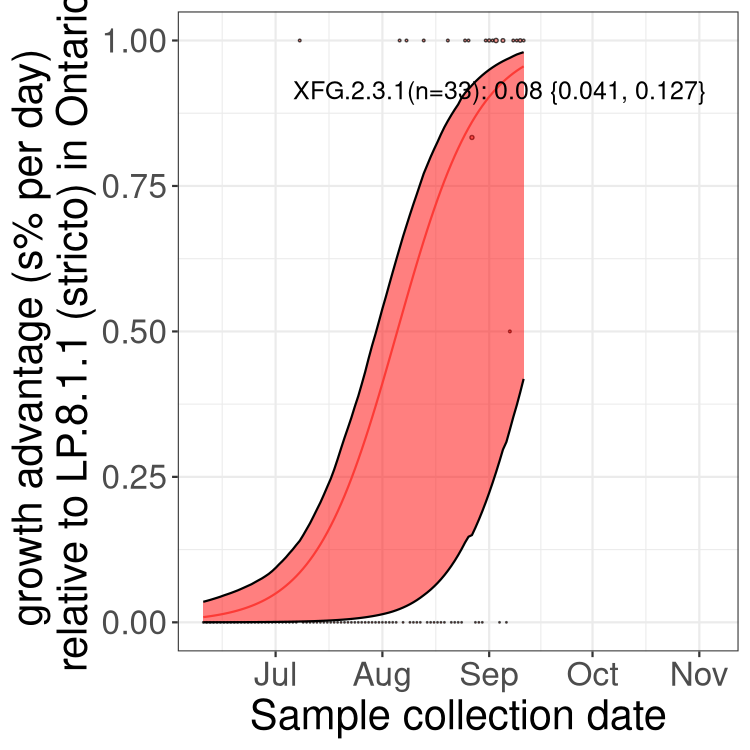
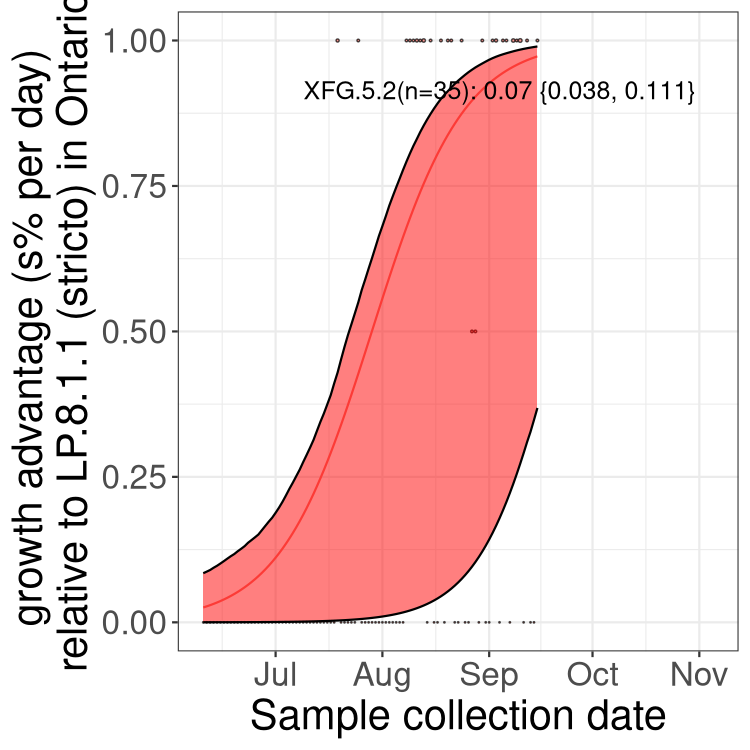
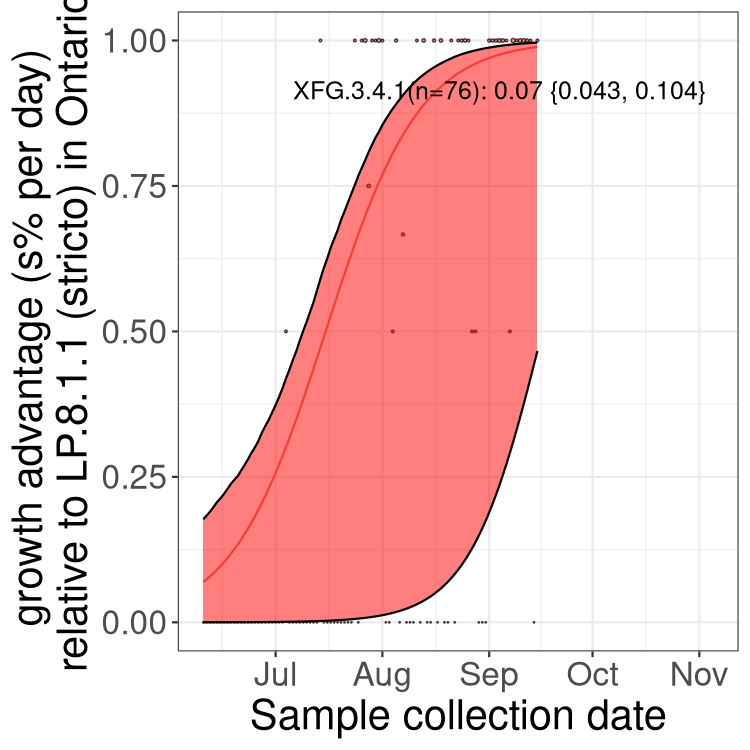
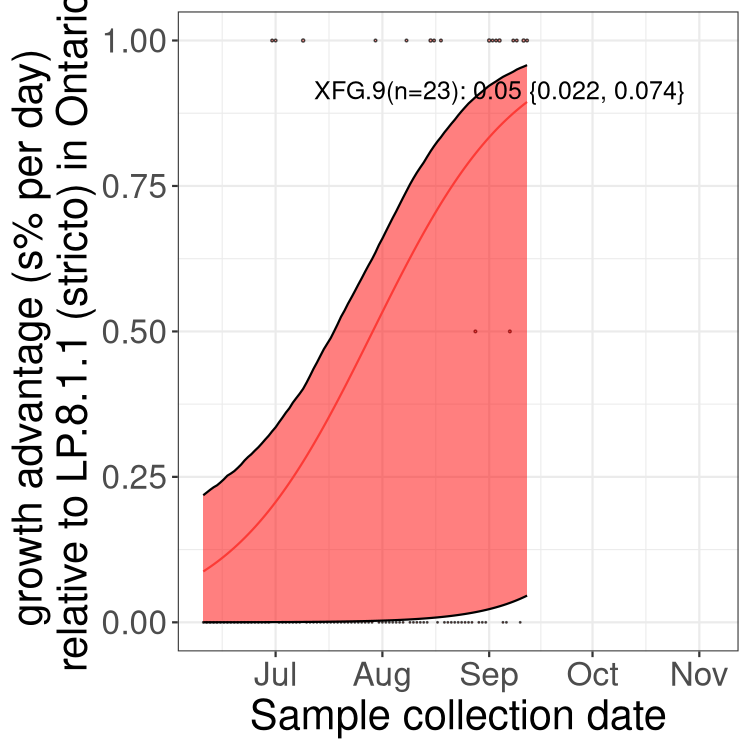
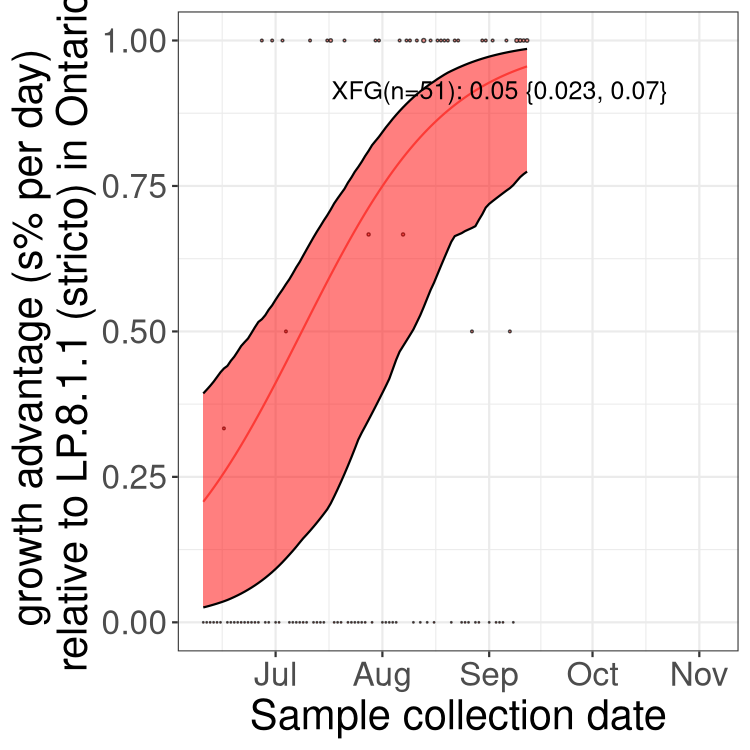
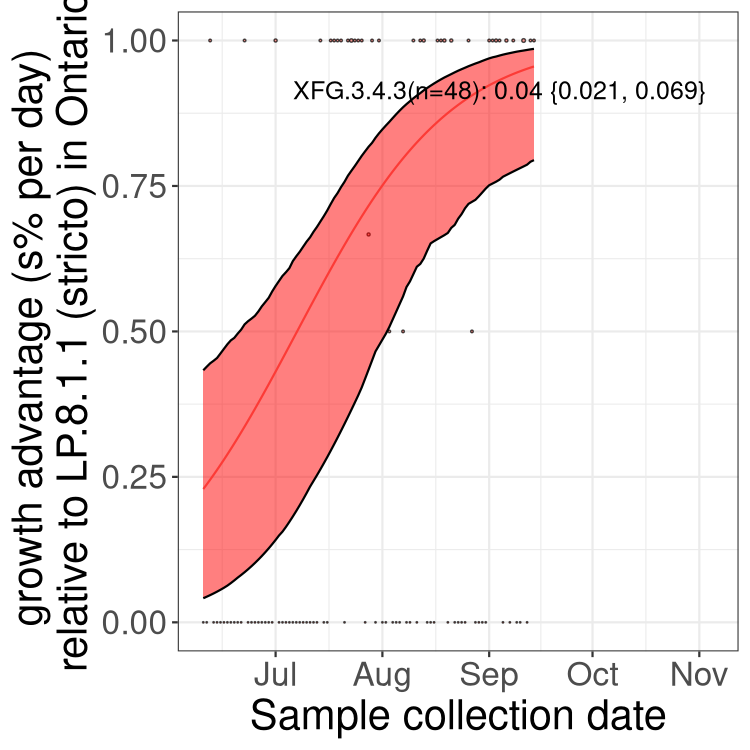
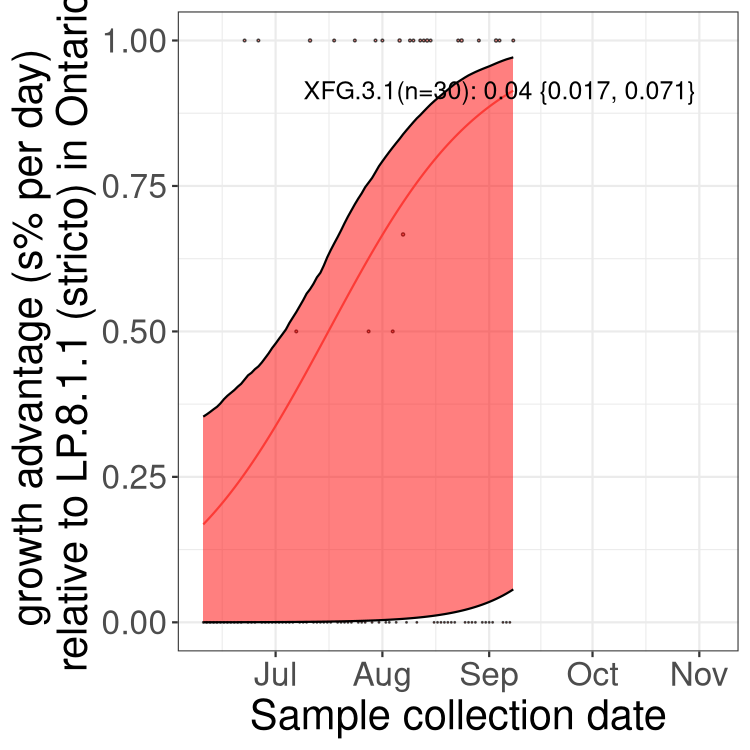
Here we show the trends of the various BA.2.* sublineages over time, excluding any recombinants, relative to the frequency of XFG.3 by itself (shown for sublineages with at least 50 (Canada) or 20 (provinces) cases). Proportions shown here are only among XFG.3 (stricto) and the lineage illustrated. Note that these plots are not necessarily representative of trends in each province and that mixing of data from different provinces may lead to shifts in frequency that are not due to selection.
Canada
Canada
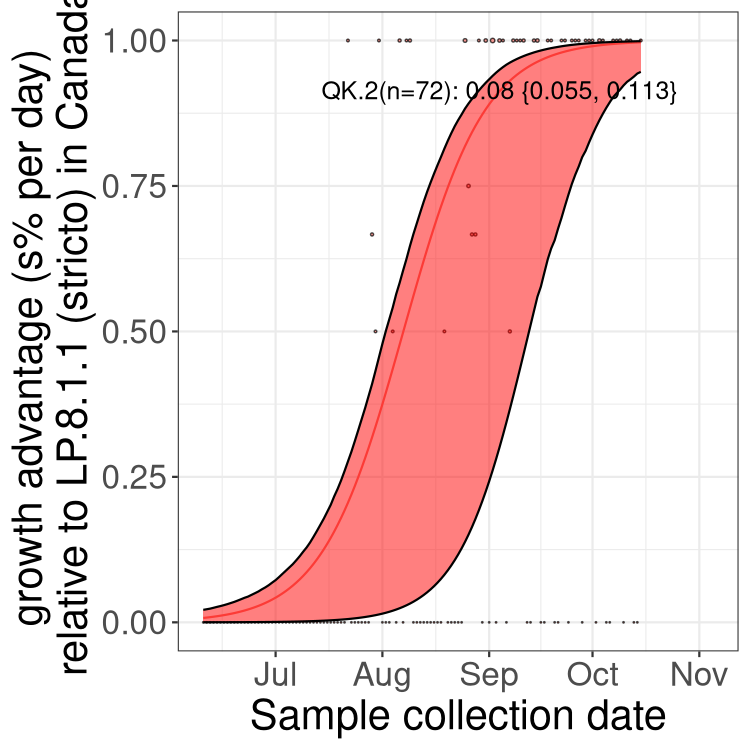
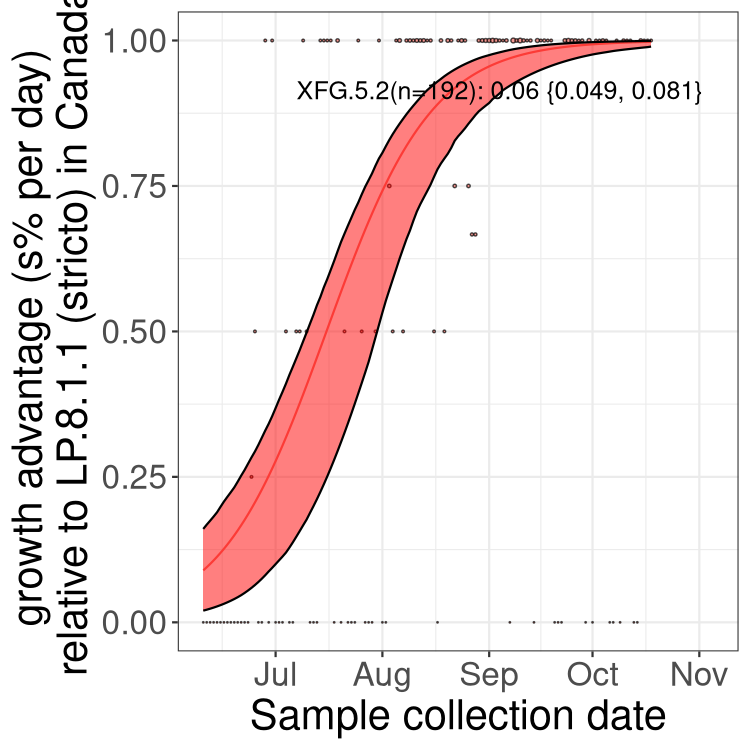
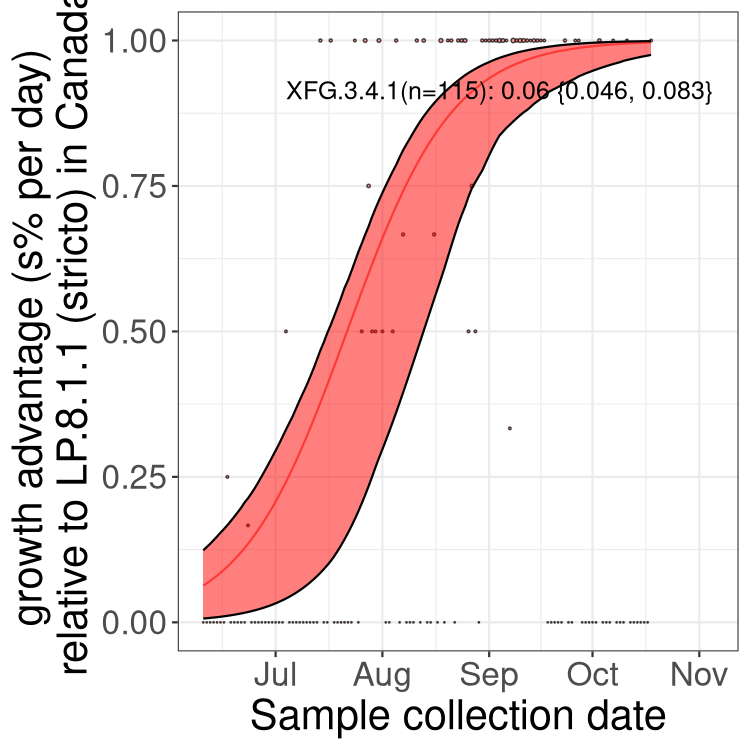
Only the three most strongly selected variants are displayed. Click here to see the rest.
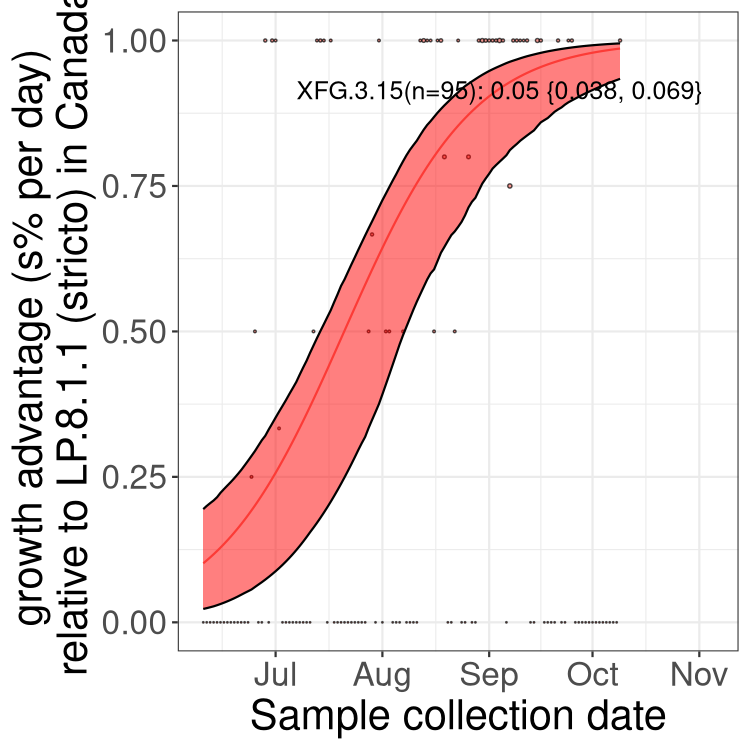
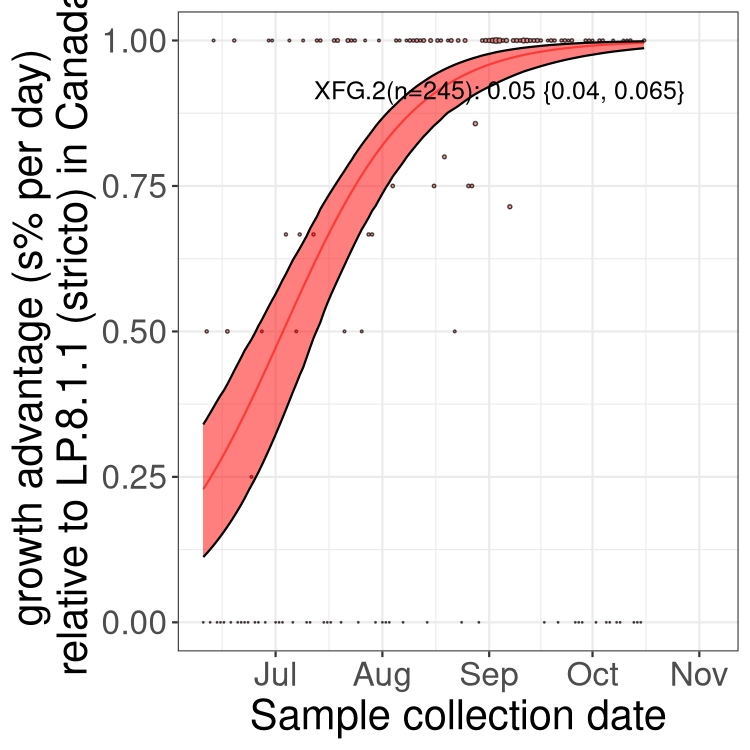
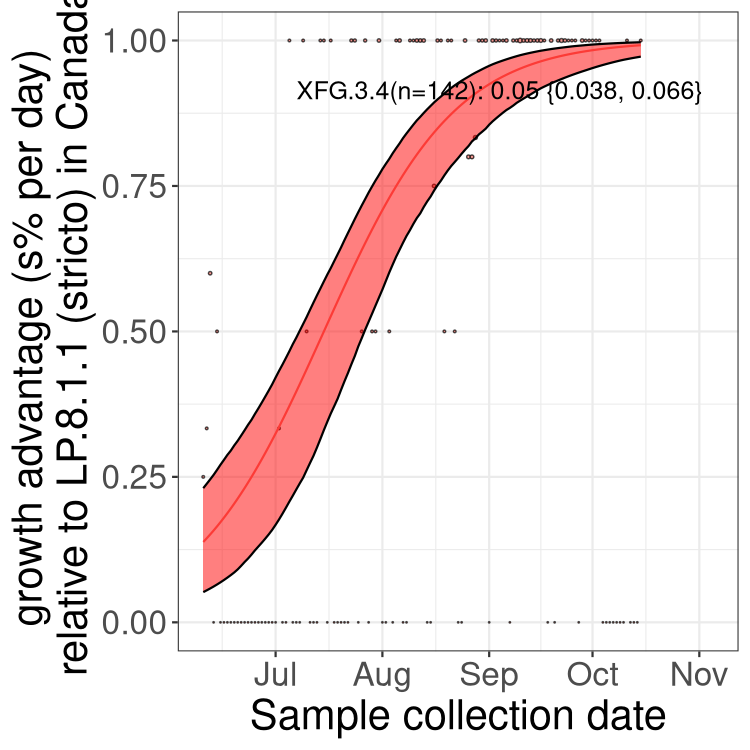
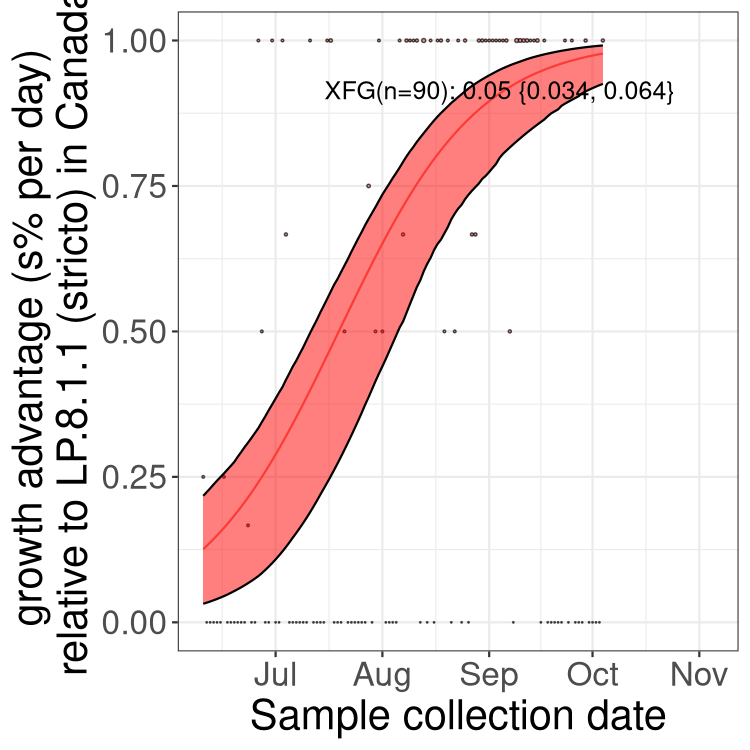
BC
British Columbia
AB
Alberta
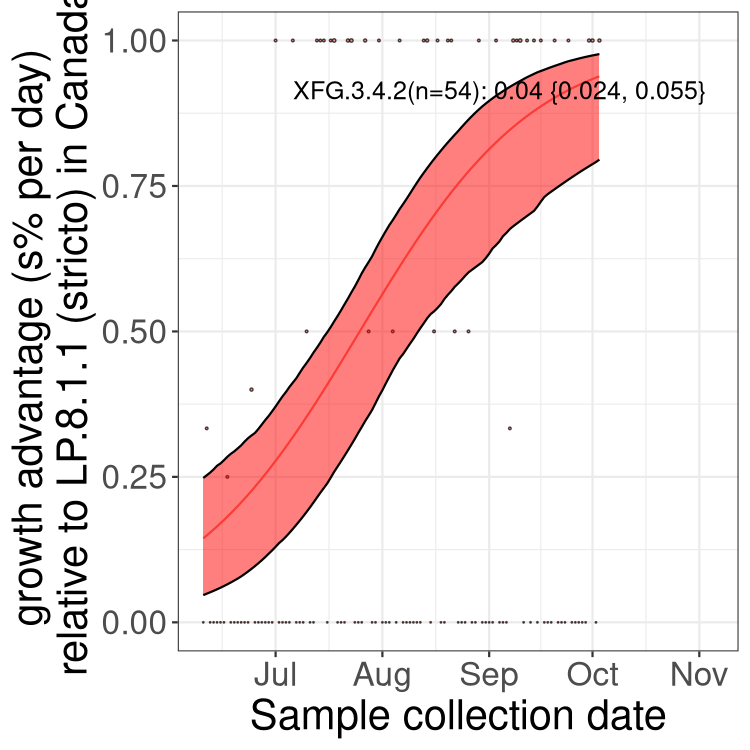
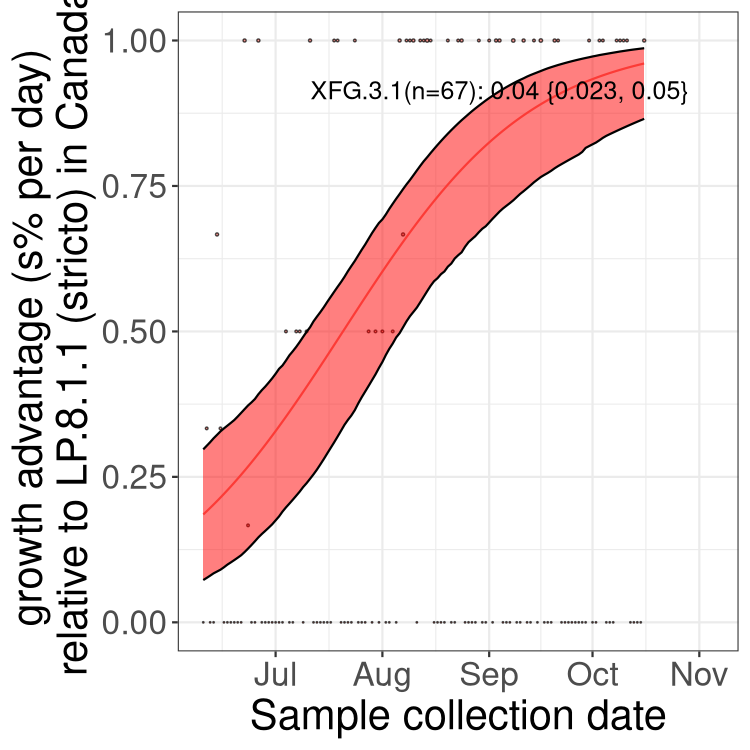
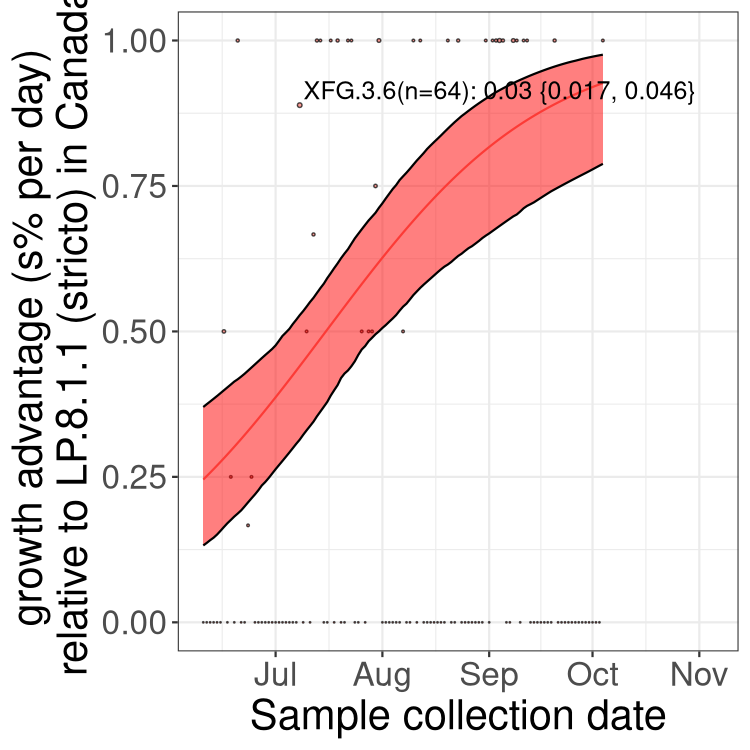
Only the three most strongly selected variants are displayed. Click here to see the rest.
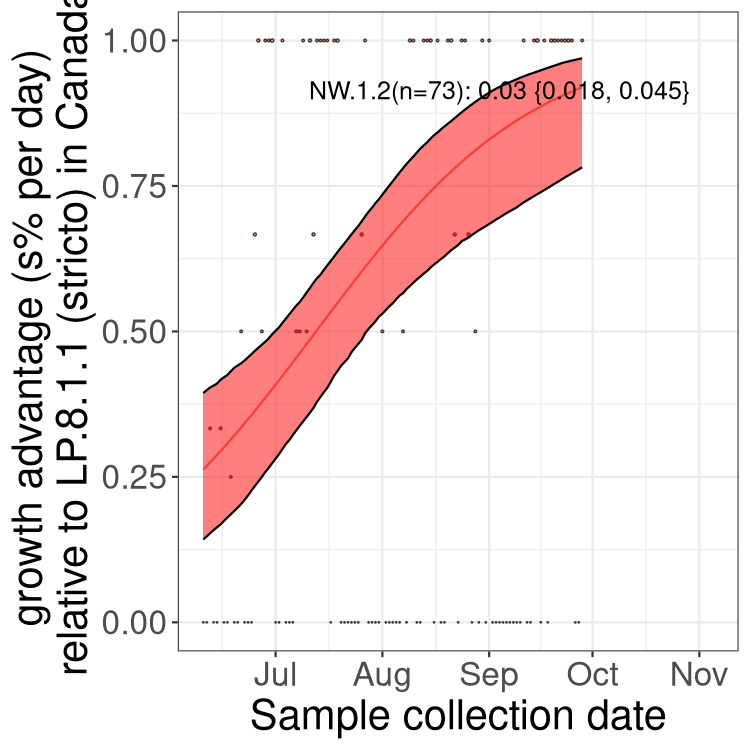
SK
Saskatchawan
MB
Manitoba
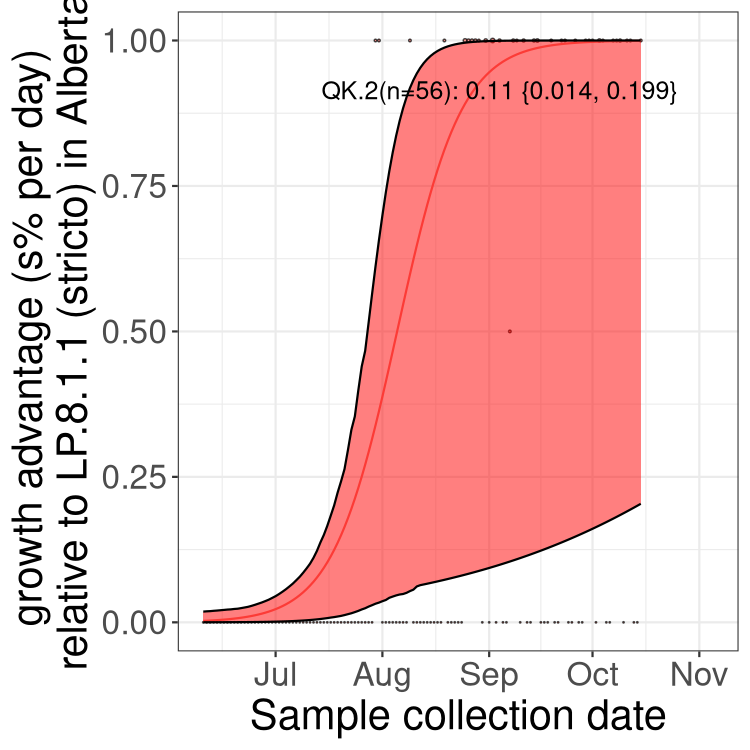
ON
Ontario
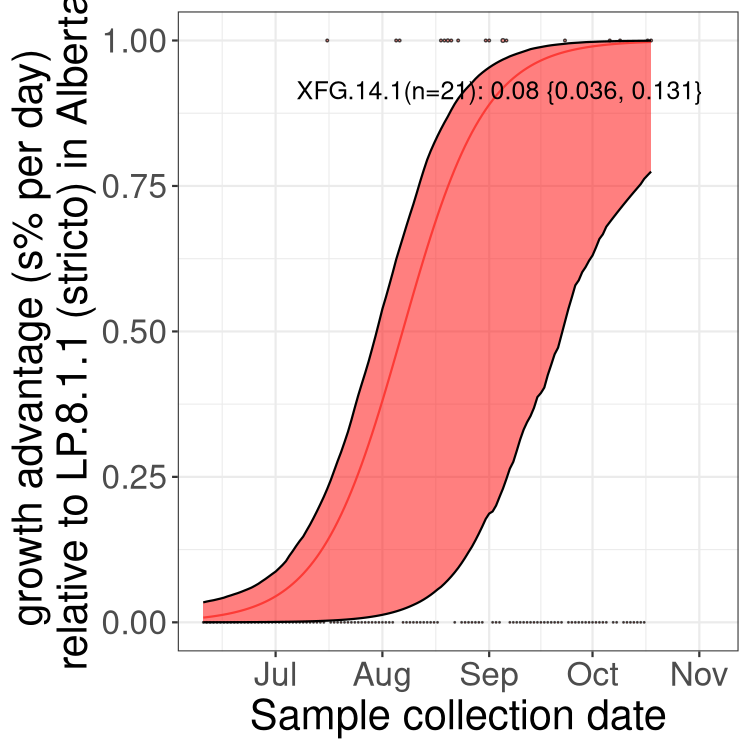
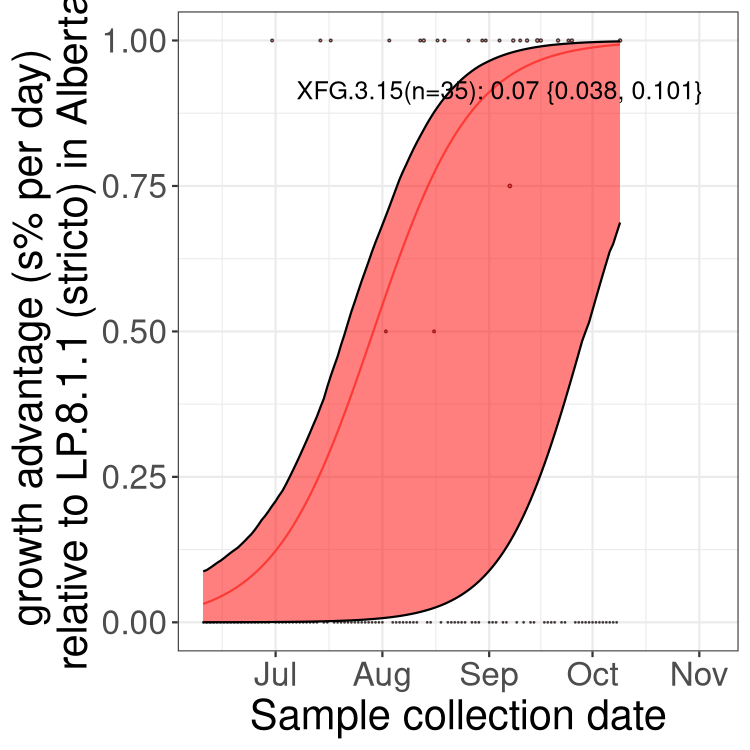
Only the three most strongly selected variants are displayed. Click here to see the rest.
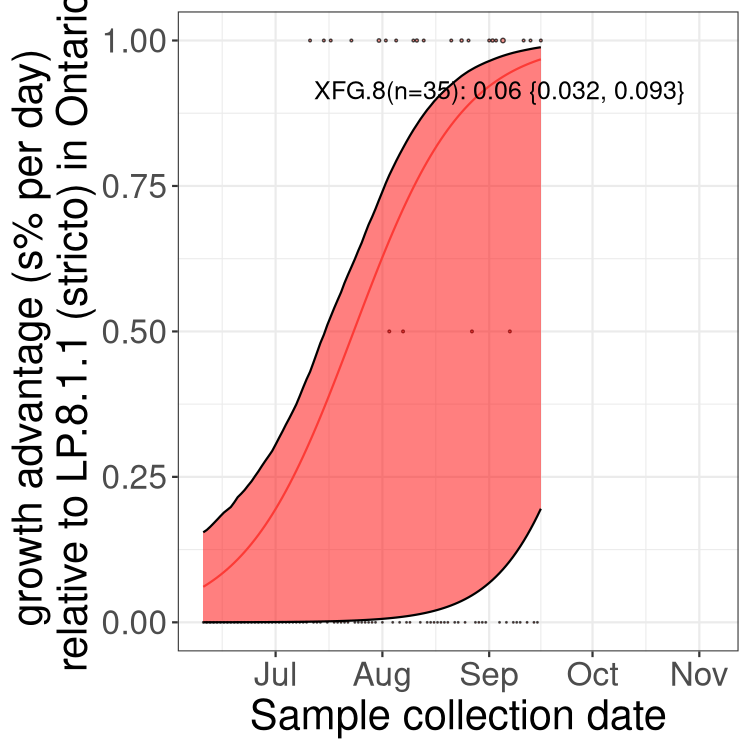
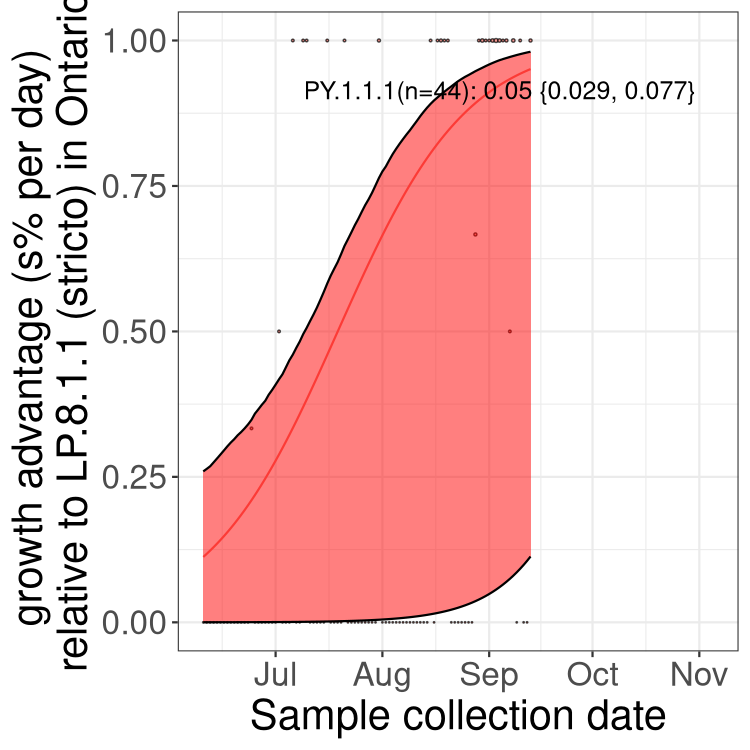
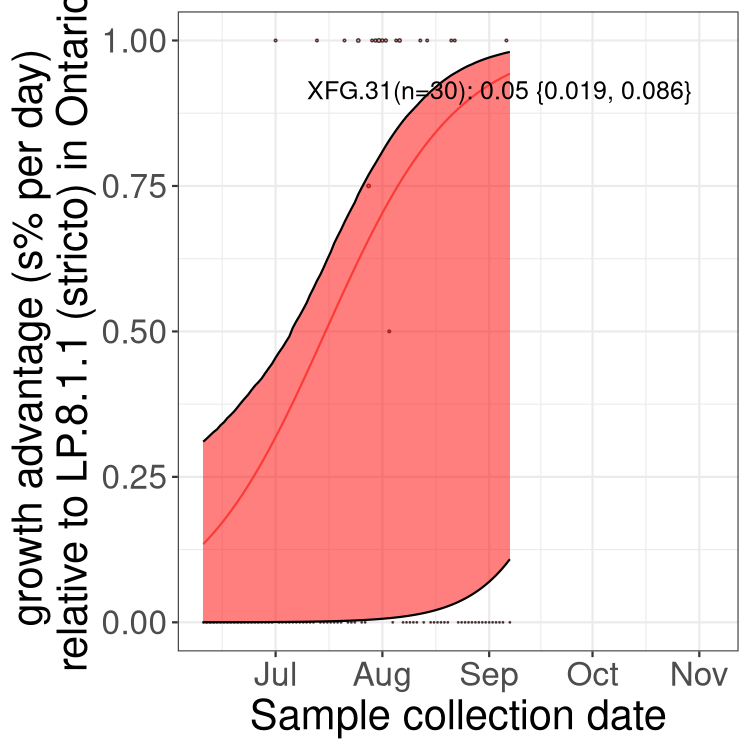
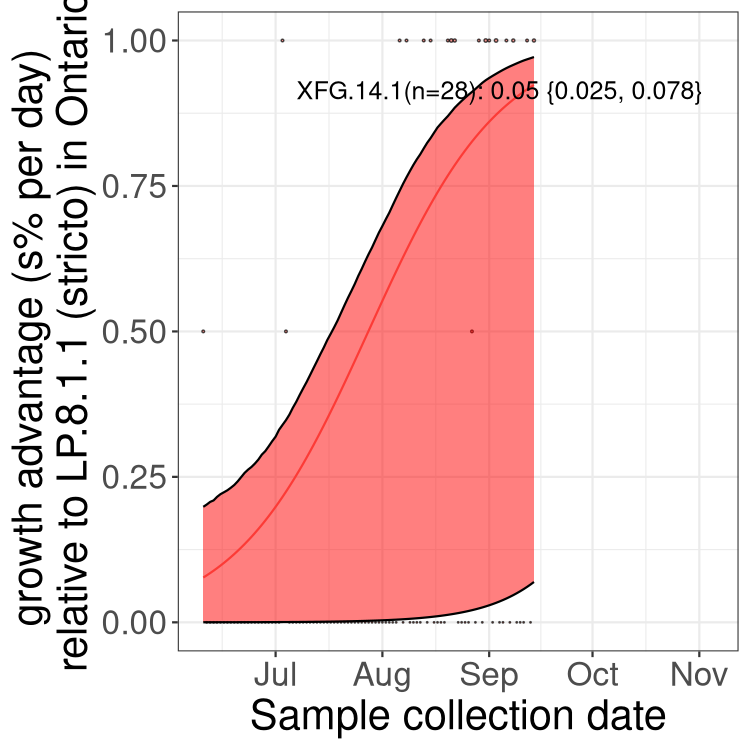
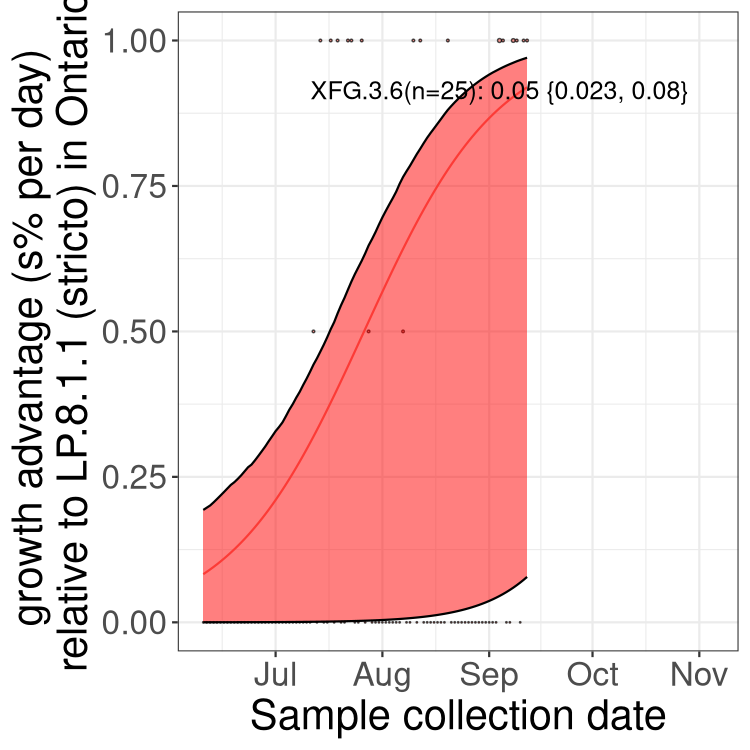
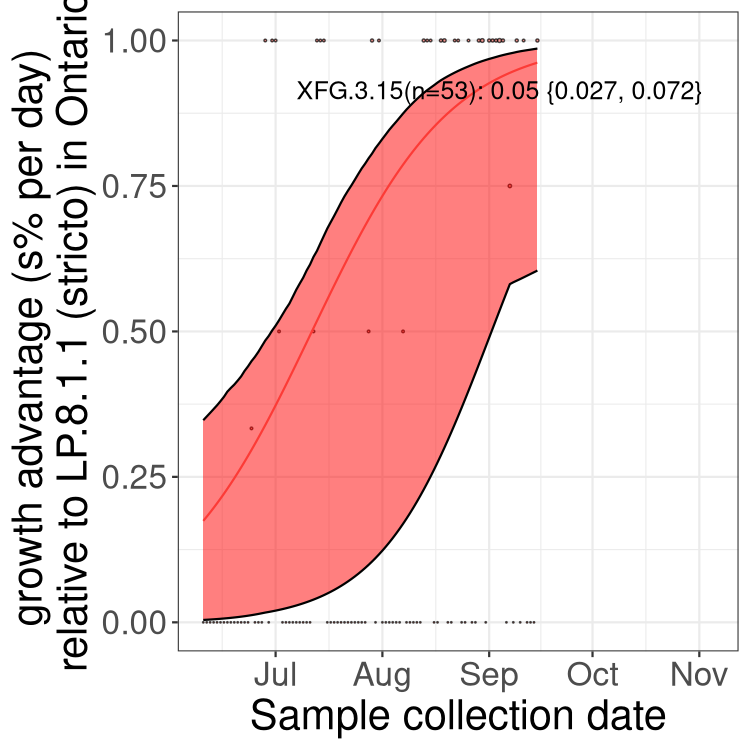
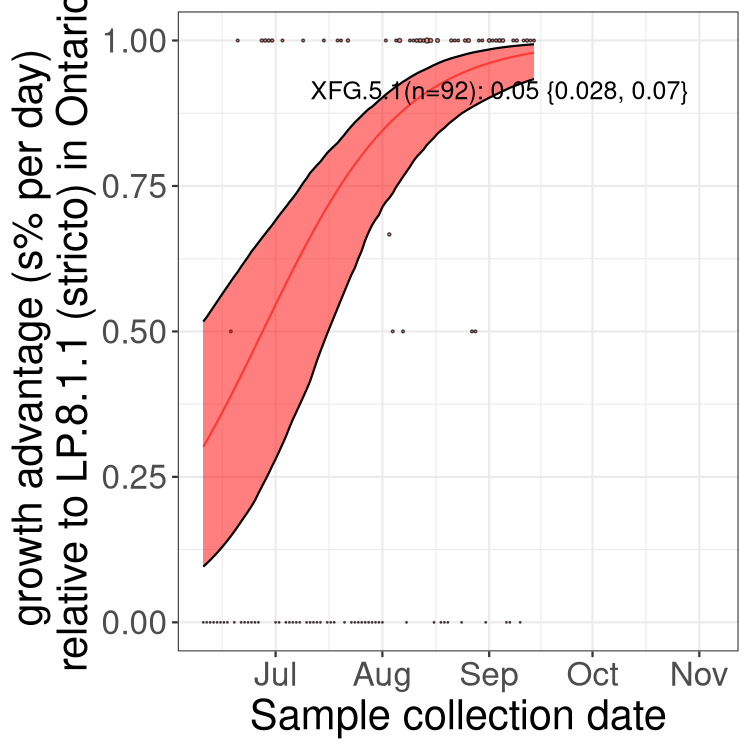
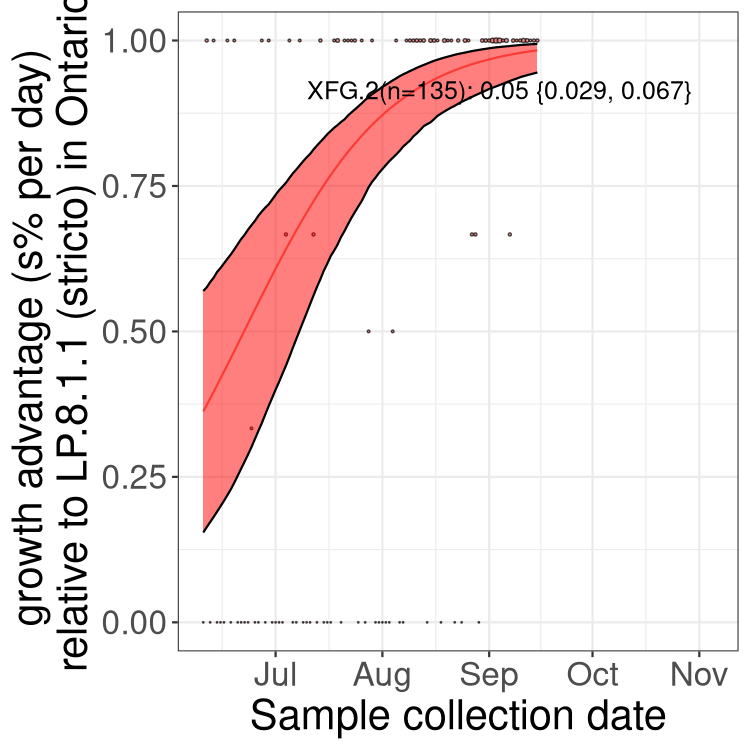
QC
Quebec
NS
Nova Scotia
NB
New Brunswick
NL
Newfoundland and Labrador
NULL
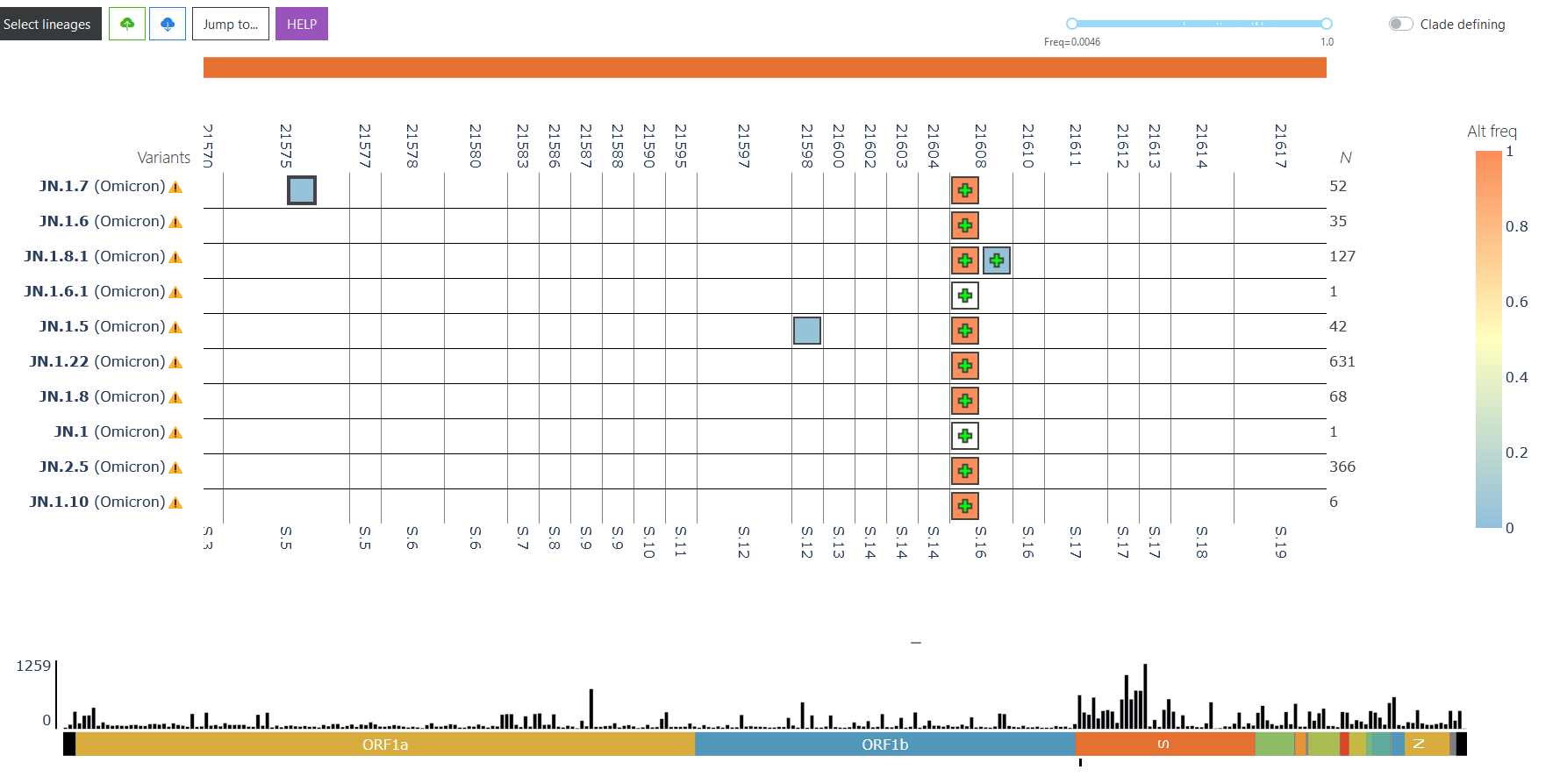
VIRUS-MVP: Mutational composition of Omicron
The image below is a screenshot from VIRUS-MVP showing a snapshot of the mutations from lineages actively circulating in Canada. Please click on the link to scan the entire genome and examine the functional impact of the mutations. More details, click on the image below or see https://virusmvp.org/covid-mvp.
Variants in Canada over time
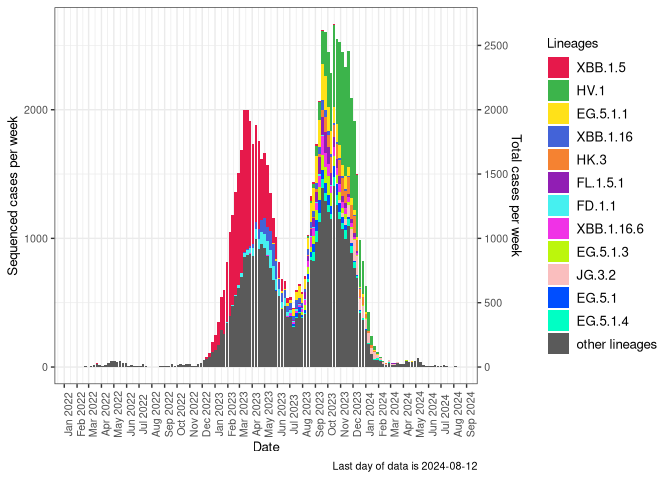
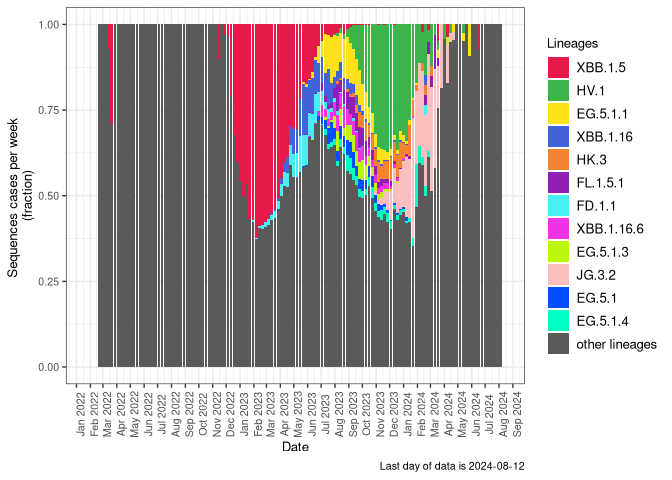
This plot shows the changing composition of sequences for all Canadian data posted to the VirusSeq Portal according to Pango lineage designation, up to 09 January, 2026. Because sampling and sequencing procedures vary by region and time, this does not necessarily reflect the true composition of SARS-CoV-2 viruses in Canada over time.
Canadian trees
Below is an interactive visualization of a subsampled phylogenetic snapshot of SARS-CoV-2 genomes from Canada. Please see methods for details.
The x-axis of the time tree represents the estimated number of years from today for which the root emerged. The x-axis of the diversity trees shows the number of mutations from the outgroup.
Hovering over a node will display a tool tip with sequence metadata, clicking on a node with the tooltip shown will copy the isolate ID to your clipboard.
### metadata and trees
source("scripts/tree.r")
# load trees from files
mltree <- read.tree(paste0(params$datadir,"/aligned_nonrecombinant_sample1.rtt.nwk"))
ttree <- read.tree(paste0(params$datadir,"/aligned_nonrecombinant_sample1.timetree.nwk"))
recombTTree <- read.tree(paste0(params$datadir,"/aligned_recombinant_X_sample1.timetree.nwk"))
recombMLree <- read.tree(paste0(params$datadir,"/aligned_recombinant_X_sample1.rtt.nwk"))
#stopifnot(all(sort(mltree$tip.label) == sort(ttree$tip.label)))
dateseq <- seq(ymd('2019-12-01'), ymd('2022-12-01'), by='3 month')
# tips are labeled with [fasta name]_[lineage]_[coldate]
# extracting just the first part makes it easier to link to metadata
mltree$tip.label <- reduce.tipnames(mltree$tip.label)
ttree$tip.label <- reduce.tipnames(ttree$tip.label)
recombTTree$tip.label <- reduce.tipnames(recombTTree$tip.label)
fieldnames<- c("fasta_header_name", "province", "host_gender", "host_age_bin",
"sample_collected_by", "purpose_of_sampling",
"lineage", "pango_group","week", "GID")
# extract rows from metadata table that correspond to ttree
metasub1 <- meta[meta$fasta_header_name%in% ttree$tip.label, fieldnames]
# sort rows to match tip labels in tree
metasub1 <- metasub1[match(ttree$tip.label, metasub1$fasta_header_name), ]
#omi tree metadata
#metasub_omi <- metasub1[grepl("Omicron",metasub1$pango_group ), ]
#recomb tree metadata
mmetasub_recomb <- meta[meta$fasta_header_name%in% recombTTree$tip.label, fieldnames]
mmetasub_recomb <- mmetasub_recomb[match(recombTTree$tip.label, mmetasub_recomb$fasta_header_name), ]
#scale to number of mutations
mltree$edge.length <- mltree$edge.length*29903
mltree <- ladderize(mltree, FALSE)
recombMLree$edge.length <- recombMLree$edge.length*29903
recombMLree <- ladderize(recombMLree, FALSE)
#enforce a non zero branch length so lines can be drawn in javascript
###Time Tree
ttree$edge.length[ttree$edge.length == 0] <- 1e-4
#ttree <- ladderize(ttree, FALSE)
recombTTree$edge.length[recombTTree$edge.length == 0] <- 1e-4
#recombTTree <- ladderize(recombTTree, FALSE)
hab=unique(meta$host_age_bin)
hab=hab[order(hab)]
months=unique(meta$month)
months=as.character(months[order(months)])
weeks=unique(meta$week)
weeks=as.character(weeks[order(weeks)])
presetColors=data.frame(name=c("other",
VOCVOI$name,
hab,
months,
weeks),
color=c("#777777",
VOCVOI$color,
rev(hcl.colors(length(hab)-1, "Berlin")),"#777777",
hcl.colors(length(months), "Berlin"),
hcl.colors(length(weeks), "Berlin")
))
#suppressWarnings({
# res <- ace(metasub1$pango.group, ttree2, type="discrete", model="ER")
#})
#idx <- apply(res$lik.anc, 1, which.max)[2:nrow(res$lik.anc)] # exclude root edge
#anc <- levels(as.factor(metasub1$pango.group))[idx]
source("scripts/tree.r")
timeTreeJsonObj <- DrawTree(ttree, metasub1, "timetree", presetColors, fieldnames=fieldnames)
recombTimeTreeJsonObj <- DrawTree(recombTTree, mmetasub_recomb, "recombtimetree", presetColors, "lineage", fieldnames= fieldnames)
#diversity ML tree
diversityTreeJsonObj <- DrawTree(mltree, metasub1, "mltree", presetColors, fieldnames=fieldnames)
recombDiversityTreeJsonObj <- DrawTree(recombMLree, mmetasub_recomb, "recombmltree", presetColors, "lineage", fieldnames=fieldnames)
#write(recombDiversityTreeJsonObj, "downloads/test.json")
### omicron diversity tree
#MLtree_omi<-keep.tip(mltree, metasub_omi$fasta_header_name)
#OmicrondiversityTreeJsonObj <- DrawTree(MLtree_omi, metasub_omi, "omimltree", presetColors, fieldnames=fieldnames)Time Tree
XBB* time tree
Diversity Tree
XBB* Diversity tree
Root-to-tip analyses
The slope of root-to-tip plots over time provide an estimate of the substitution rate. A lineage with a steeper positive slope than average for SARS-CoV-2 is accumulating mutations at a faster pace, while a lineage that exhibits a jump up (a shift in intercept but not slope) has accumulated more than expected numbers of mutations in a transient period of time (similar to what we saw with Alpha when it first appeared in the UK).
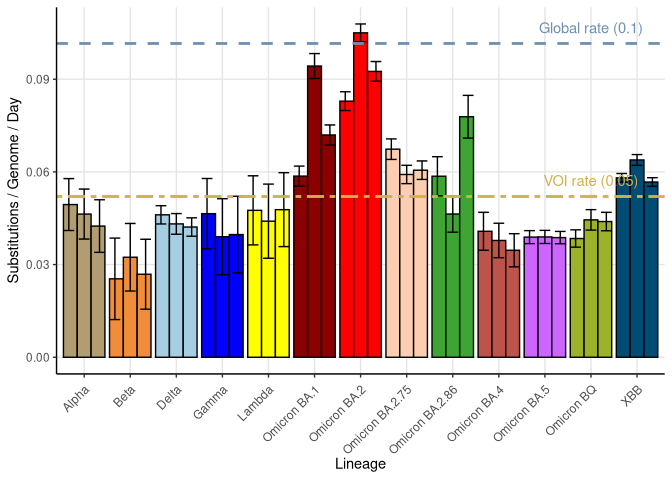
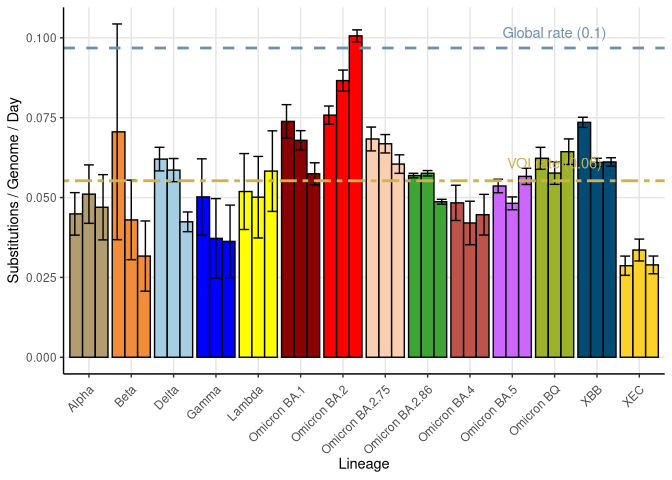
Molecular clock estimates (based on three independent subsamples)
Here we show the estimate of the substitution rate for 3 independent subsamples of different variants of interest (VOI), with their 95% confidence interval. The average rate of substitution within VOI is given by a bamboo colored dashed line. For comparison, the average rate of substitution across all samples is much higher (grey line), indicating that about half of the substitutions occur through normal routes (i.e. not chronic infections) of transmission while the other half occur with the appearance of new, highly divergent VOI, likely due to different evolutionary processes occurring within chronic infections (see Neher 2022 for details). BA.2 (red) appears to have a higher rate of mutation because it includes highly divergent sub-groups (i.e., potential saltation lineages), including CM.* and sub-types related to BA.2.86.
Lineages
Here we present a searchable table that provides a short description of each PANGO lineage, it’s immediate ancestor, and the number of sequences in the last 120days.
Appendix
Future development
We are in the process of adding or would like to develop code for some of the following analyses:
- dN/dS (by variant and by gene/domains)
- Tajima’s D over time
- clustering analyses
- genomically inferred epidemiological parameters: R0, serial interval, etc.
With anonymized data on vaccination status, severity/outcome, reason for sequencing (e.g., outbreak, hospitalization, or general sampling), and setting (workplace, school, daycare, LTC, health institution, other), we could analyze genomic characteristics of the virus relative to the epidemiological and immunological conditions in which it is spreading and evolving. Studies on mutational correlations to superspreading events, vaccination status, or comparisons between variants would allow us to better understand transmission and evolution in these environments.
List of useful tools
A selection of bioinformatics, phylogenetic, and modelling tools that are useful for SARS-CoV-2 analyses:
- UShER: Ultrafast Sample placement on Existing tRee - for placing a small-ish dataset into the global GISAID phylogenetic tree.
- List of (mostly) modelling tools by CANMOD, includes RECON, an outbreak tools for both modelling and genomic epidemiology
- The Epi Graph Network: training platform. Programming tools for health data analysis, African/European network of researchers and WHO Afro.
- Pokay tool for checking and reporitng mismatches
- IRIDA Canada’s ID analysis platform for genomic epidemiology
- cov-lineages: summaries of Pango lineages
- CoVizu: analysis and visualization of the global diversity of SARS-CoV-2 genomes in real time
- VIRUS-MVP: mutation tracker and visualization in real-time from Centre for Infectious Disease Genomics and One Health (CIDGOH)
- Outbreak Info: SARS-CoV-2 data explorer: lineage comparison, mutation tracker, etc
- Mike Honey’s SARS-CoV-2 genomes DataViz Projects
- Raj Rajnarayanan’s data “vizzes”
- SMDP: SARS-CoV-2 Mutation Distribution Profiler
We look for additional combinations of mutations identified through mutation scanning that are involved in binding or immune evasion. See for example:
- Greaney, Starr, & Bloom, Virus Evolution, 8:veac021 (2022)
- Cao et al, Nature, 614:521-529 (2023)
- Yisimayi et al, bioRxiv, DOI 10.1101/2023.05.01.538516 (2023)
- Dadonaite et al, bioRxiv, DOI 10.1101/2023.11.13.566961 (2023)
- Bdeir et al, medRxiv, DOI 10.1101/2024.01.03.23300575 (2024)
Methodology
Genome data and metadata are sourced from the Canadian VirusSeq Data Portal. Pango lineage assignments are generated using the pangoLEARN algorithm. Source code for generating this RMarkdown notebook can be found in [https://github.com/CoVaRR-NET/duotang].
Trees
Phylogenetic trees
Canadian genomes were obtained from the VirusSeq data on the February 09, 2026 and down-sampled to two genomes per lineage, province and month before October 2021, and five genomes per lineage, province and month after October 2021 (about 10,000 genomes in total). We used a Python wrapper of minimap2 (version 2.17) to generate multiple sequence alignments for these genome samples. A maximum likelihood (ML) tree was reconstructed from each alignment using the COVID-19 release of IQ-TREE (version 2.2.0). Outliers were identified in by root-to-tip regression using the R package ape and removed from the dataset. TreeTime was used to reconstruct a time-scaled tree under a strict molecular clock model. The resulting trees were converted into interactive plots with ggfree and r2d3.
Selection
Selection Coefficents
To estimate selection, we used standard likelihood techniques. In brief, sublineages of interest were prespecified (e.g., JN.1) and counts by day tracked over time. If selection were constant over time, the frequency of sub-type \(i\) at time \(t\) would be expected to rise according to \[p_i(t) = \frac{p_i(0) \exp(s_i t)}{\sum_j p_j(0) \exp(s_j t)},\] where \(s_i\) is the selection coefficient favouring sub-type \(i\). A selection coefficient of \(s_i=0.1\) implies that sub-type \(i\) is expected to rise from 10% to 90% frequency in 44 days (in \(4.4./s_i\) days for other values of \(s_i\)).
At any given time \(t\), the probability of observing \(n_i\) sequences of sublineage \(i\) is multinomially distributed, given the total number of sequences from that day and the frequency of each \(p_i(t)\). Consequently, the likelihood of seeing the observed sequence data over all times \(t\) and over all sublineages \(j\) is proportional to \[L = \prod_t \prod_j p_i(t)^{n_i(t)}.\]
The BBMLE package in R was used to maximize the likelihood of the observed data (using the default optimization method, optim). For each selection coefficient, 95% confidence intervals were obtained by profile likelihood (using uniroot).
Graphs illustrating the rise in frequency of a variant over time are shown (left panels), with the area of each dot proportional to the number of sequences. 95% confidence bands were obtained by randomly drawing 10,000 sets of parameters (\(p_i\) and \(s_i\) for each sub-type) using RandomFromHessianOrMCMC, assuming a multi-normal distribution around the maximum likelihood point (estimated from the Hessian matrix, Pawitan 2001). At each point in time, the 2.5%-97.5% range of values for \(p_i(t)\) are then shown in the confidence bands.
Logit plots (right panels) show \[ln(\frac{p_i(t)}{p_{ref}(t)})\] relative to a given reference genotype (here BA.1), which gives a line whose slope is the strength of selection \(s_i\). Changes in slope indicate changes in selection on a variant (e.g., see Otto et al.).
These estimates of selection ignore heterogeneity within provinces and may be biased by the arrival of travel-related cases while frequencies are very low. Sampling strategies that oversample clustered cases (e.g., sequencing outbreaks) will introduce additional variation beyond the multinomial expectation, but these should lead to one-time shifts in frequency rather than trends over time. Provinces with sampling strategies that are variant specific are removed, unless explicit information about the variant frequencies is available.
Detection trends by variant
As of 13 July 2024, the number of detected cases for all provinces are obtained from the “Respiratory virus detection data” (PHAC; link), multiplying the number of SARS-CoV-2 tests times the fraction that are positive. These reported counts are then normalized to cases per 100,000 individual based off of Statistics Canada’s population estimates for each province or the total population of Canada. We then remove the last 1 data point as those data continue to be gathered and are likely underestimated. This normalized cases over time (\(n\)) is then log transformed and fitted to a smooth spline with a lambda value of 0.001 using the R stats’ smooth.spline() function. A list of smoothed detection counts (\(n(t)\)), where each element correspond to a count on a particular date \(t\), is then obtained from this fitting function by reversing the log transformation.
The previously discussed methods allow us to estimate the proportion of a lineage over time (see equation for \(p_i(t)\) above). This produces a list of proportions for each lineage of interest, where each element correspond to a lineage proportion on a particular date, \(t\). By multiplying the list from detection counts to this lineage frequency list at all time points, we can estimate the inferred number of reported cases that are due to a specific lineage at each time point as \[n_i(t) = p_i(t) \; n(t).\]
Finally, once the inferred detection counts (\(n_i(t)\)) of each lineages are calculated, we take the last two days of data from the smooth spline times lineage frequency curve to get \(n_i(t)\) and \(n_i(t-1)\), from which the growth rate of that lineage (\(r_i\)) is estimated as \[r_i = ln\left( \frac{n_i(t)}{n_i(t-1)} \right).\]
Because genomics data often lag detection count data, the last date of reliable detection count data may be closer to the present than the last date of genomics data. In these cases, the evolutionary model for \(p_i(t)\) is projected forward in time and shown as a ligher shaded area under the curve within the plot).
Rates
Root-to-tip estimates of substitution rate
Substitution rates were obtained from the maximum likelihood tree made using IQ-TREE and a root-to-tip regression conducted, without forcing the intercept to zero (similar results were seen when forcing the intercept). Up to 10000 samples for non-XBB lineages and all samples for XBB lineages are used to construct this tree. For the estimation of each VOI’s substitution rates over time, all sequences of that VOI present in the tree are used. While this ignores pseudo-replication among the samples due to relatedness, the estimated slope is robust given the large sample sizes. Furthermore, we calculated SE bars from three different independent samples to reduce the influence of closely related viral samples. The global rate estimate was obtained by a regression over time of all the sequences present in the tree, as illustrated in the root-to-tip plot, ignoring variants classification.
Data notes by province
All analyses draw on the most recent publicly available viral sequence data on ViralSeq and should be interpreted with caution due to lags in reporting and sequencing priorities that can differ across provinces or territories. Note that the NCCID provides a timeline of Canadian events related to each variant: https://nccid.ca/covid-19-variants/.
As of 13 July 2024, the number of detected cases is obtained from the “Respiratory virus detection data” (PHAC; link).
Historical notes
From the beginning of the pandemic to the fall of 2021, Canadian sequences were mostly of the wildtype lineages (pre-VOCs). By the beginning of summer 2021, the VOCs Alpha and Gamma were the most sequenced lineages overall in Canada. The Delta wave grew during the summer of 2021 with sublineages AY.25 and AY.27 constituting sizeable proportions of this wave. Omicron arrived in November of 2021 and spread in three main waves, first BA.1* (early 2022), then BA.2* (spring 2022), then BA.5* (summer 2022). Current, multiple sublineages of Omicron persist, with emerging sublineages spreading, such as BQ.1.1 (a BA.5 sub-lineage).
There are two Pango lineages that have a Canadian origin and that predominately spread within Canada (with some exportations internationally): B.1.438.1 and B.1.1.176. Other lineages of historical interest in Canada:
- A.2.5.2 - an A lineage (clade 19B) that spread in Quebec, involved in several outbreaks before Delta arrived (see this post for more details: https://virological.org/t/recent-evolution-and-international-transmission-of-sars-cov-2-clade-19b-pango-a-lineages/711)
- B.1.2 - a USA lineage that spread well in Canada
- B.1.160 - an European lineages that spread well in Canada
This historical analysis is not being further updated, as we focus on more interactive data plots and the “Current situation” text above.
Previous Versions
Session info
The version numbers of all packages in the current environment as well as information about the R install is reported below.
Hide
Show
sessionInfo()## R version 4.2.3 (2023-03-15)
## Platform: x86_64-redhat-linux-gnu (64-bit)
## Running under: Fedora Linux 37 (Server Edition)
##
## Matrix products: default
## BLAS/LAPACK: /usr/lib64/libflexiblas.so.3.3
##
## locale:
## [1] LC_CTYPE=en_CA.UTF-8 LC_NUMERIC=C
## [3] LC_TIME=en_CA.UTF-8 LC_COLLATE=en_CA.UTF-8
## [5] LC_MONETARY=en_CA.UTF-8 LC_MESSAGES=en_CA.UTF-8
## [7] LC_PAPER=en_CA.UTF-8 LC_NAME=C
## [9] LC_ADDRESS=C LC_TELEPHONE=C
## [11] LC_MEASUREMENT=en_CA.UTF-8 LC_IDENTIFICATION=C
##
## attached base packages:
## [1] stats4 grid splines parallel stats graphics grDevices
## [8] utils datasets methods base
##
## other attached packages:
## [1] HelpersMG_5.8 Matrix_1.5-3 coda_0.19-4 rlang_1.1.6
## [5] MASS_7.3-58.2 bbmle_1.0.25 plotly_4.10.1 DT_0.27
## [9] reshape2_1.4.4 forcats_1.0.0 stringr_1.5.0 dplyr_1.1.0
## [13] purrr_1.0.1 readr_2.1.3 tibble_3.1.8 tidyverse_1.3.2
## [17] jsonlite_2.0.0 r2d3_0.2.6 ggfree_0.1.0 ape_5.8-1
## [21] ggplot2_3.4.0 lubridate_1.9.1 knitr_1.50 tidyr_1.3.0
##
## loaded via a namespace (and not attached):
## [1] nlme_3.1-162 fs_1.6.6 bit64_4.0.5
## [4] httr_1.4.4 numDeriv_2016.8-1.1 tippy_0.1.0
## [7] tools_4.2.3 backports_1.4.1 bslib_0.9.0
## [10] utf8_1.2.2 R6_2.5.1 DBI_1.1.3
## [13] lazyeval_0.2.2 colorspace_2.1-0 withr_3.0.2
## [16] tidyselect_1.2.0 bit_4.0.5 compiler_4.2.3
## [19] cli_3.6.5 rvest_1.0.3 xml2_1.3.3
## [22] labeling_0.4.2 sass_0.4.10 scales_1.2.1
## [25] mvtnorm_1.1-3 digest_0.6.37 rmarkdown_2.30
## [28] pkgconfig_2.0.3 htmltools_0.5.8.1 dbplyr_2.3.0
## [31] fastmap_1.2.0 htmlwidgets_1.6.1 readxl_1.4.1
## [34] rstudioapi_0.14 farver_2.1.1 jquerylib_0.1.4
## [37] generics_0.1.3 crosstalk_1.2.0 vroom_1.6.1
## [40] googlesheets4_1.0.1 magrittr_2.0.3 Rcpp_1.1.0
## [43] munsell_0.5.0 fansi_1.0.4 lifecycle_1.0.4
## [46] stringi_1.7.12 yaml_2.3.7 plyr_1.8.8
## [49] bdsmatrix_1.3-6 crayon_1.5.2 lattice_0.20-45
## [52] haven_2.5.1 hms_1.1.2 pillar_1.8.1
## [55] reprex_2.0.2 glue_1.6.2 evaluate_1.0.5
## [58] data.table_1.14.6 modelr_0.1.10 vctrs_0.6.5
## [61] tzdb_0.3.0 cellranger_1.1.0 gtable_0.3.1
## [64] assertthat_0.2.1 cachem_1.0.6 xfun_0.54
## [67] broom_1.0.3 googledrive_2.0.0 viridisLite_0.4.1
## [70] gargle_1.3.0 timechange_0.2.0 ellipsis_0.3.2Acknowledgements
We thank all the authors, developers, and contributors to the VirusSeq database for making their SARS-CoV-2 sequences publicly available. We especially thank the Canadian Public Health Laboratory Network, academic sequencing partners, diagnostic hospital labs, and other sequencing partners for the provision of the Canadian sequence data used in this work. Genome sequencing in Canada was supported by a Genome Canada grant to the Canadian COVID-19 Genomic Network (CanCOGeN).
We gratefully acknowledge all the Authors, the Originating laboratories responsible for obtaining the specimens, and the Submitting laboratories for generating the genetic sequence and metadata and sharing via the VirusSeq database, on which this research is based.
The Canadian VirusSeq Data Portal (https://virusseq-dataportal.ca) We wish to acknowledge the following organisations/laboratories for contributing data to the Portal: Canadian Public Health Laboratory Network (CPHLN), CanCOGGeN VirusSeq, Saskatchewan - Roy Romanow Provincial Laboratory (RRPL), Nova Scotia Health Authority, Alberta Precision Labs (APL), Queen’s University / Kingston Health Sciences Centre, National Microbiology Laboratory (NML), Institut National de Sante Publique du Quebec (INSPQ), BCCDC Public Health Laboratory, Public Health Ontario (PHO), Newfoundland and Labrador - Eastern Health, Unity Health Toronto, Ontario Institute for Cancer Research (OICR), Provincial Public Health Laboratory Network of Nova Scotia, Centre Hospitalier Universitaire Georges L. Dumont - New Brunswick, and Manitoba Cadham Provincial Laboratory. Please see the complete list of laboratories included in this repository.
Public Health Agency of Canada (PHAC) / National Microbiology Laboratory (NML) - (https://health-infobase.canada.ca/covid-19/epidemiological-summary-covid-19-cases.html)
Various provincial public health websites (e.g. INSPQ https://www.inspq.qc.ca/covid-19/donnees/)
Canadian Institutes of Health Research (CIHR) - Coronavirus Variants Rapid Response Network (CoVaRR-Net;https://covarrnet.ca/)